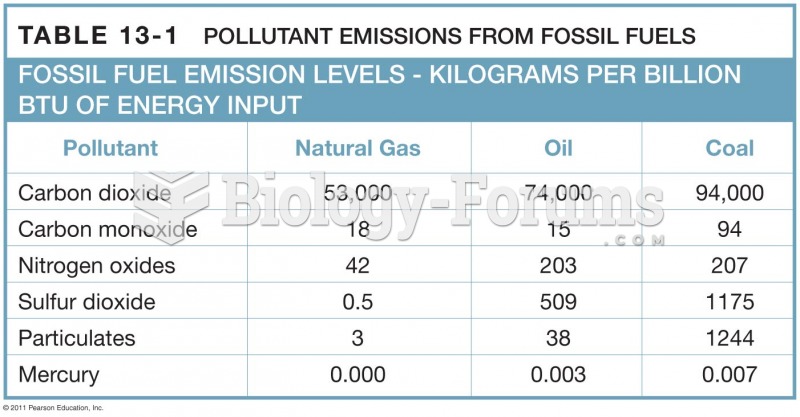
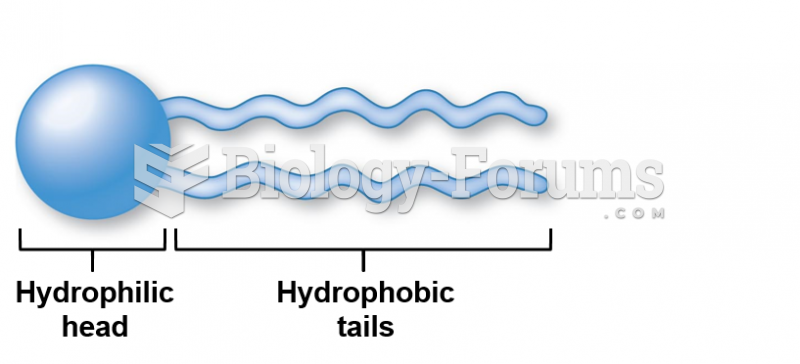
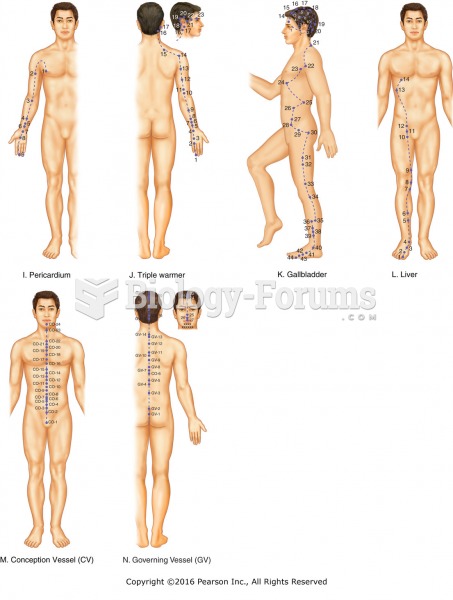
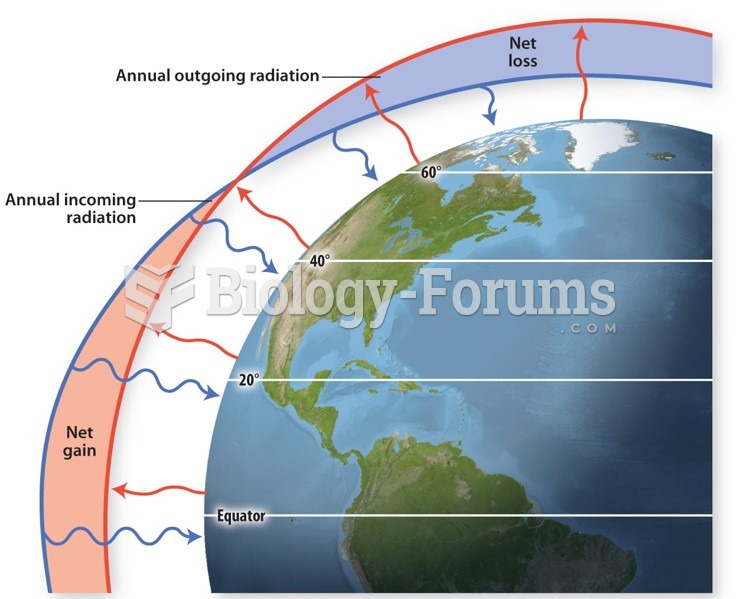
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
Did you know?
The FDA recognizes 118 routes of administration.