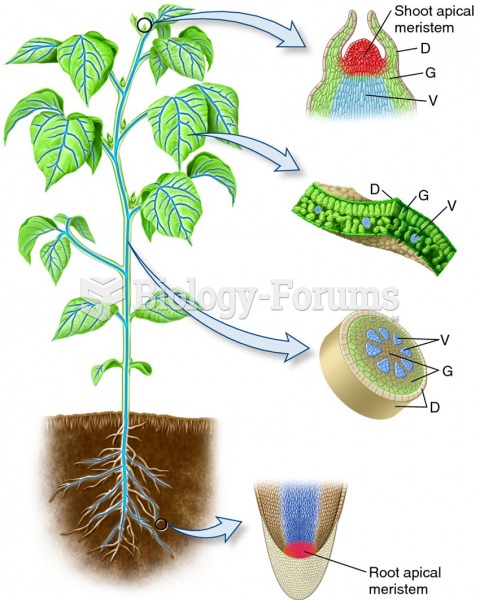
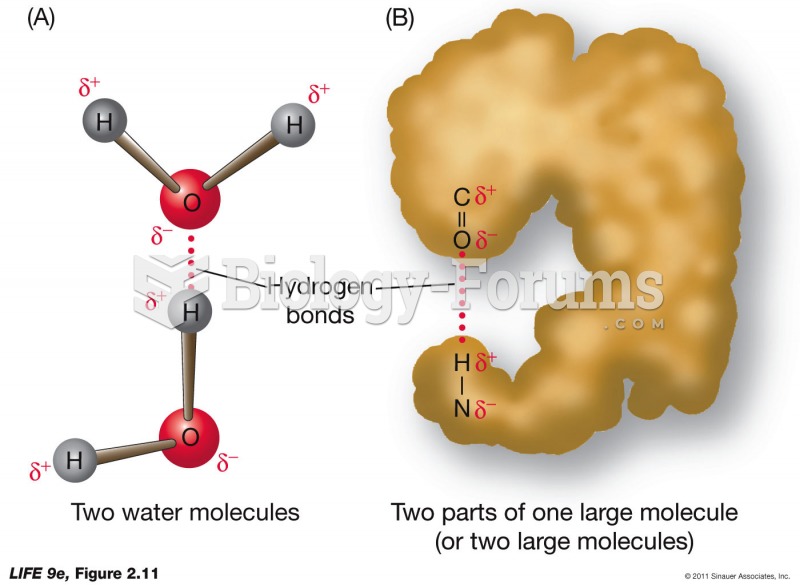
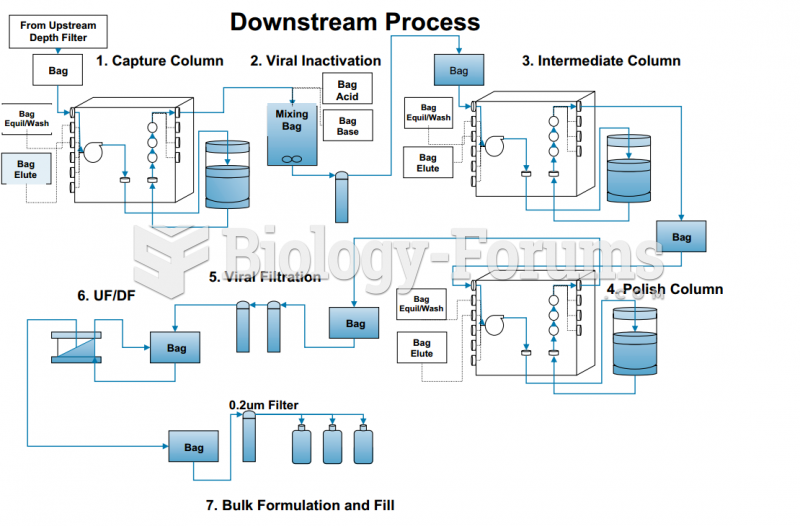
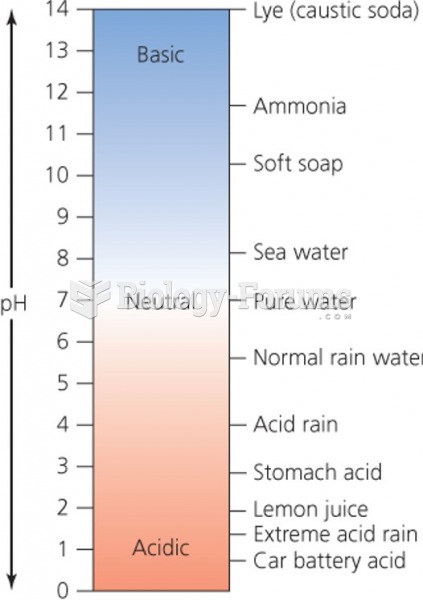
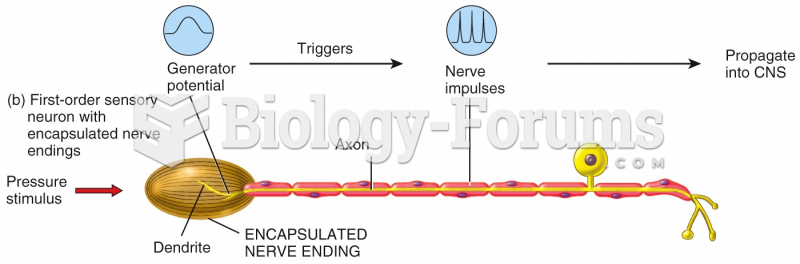
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.