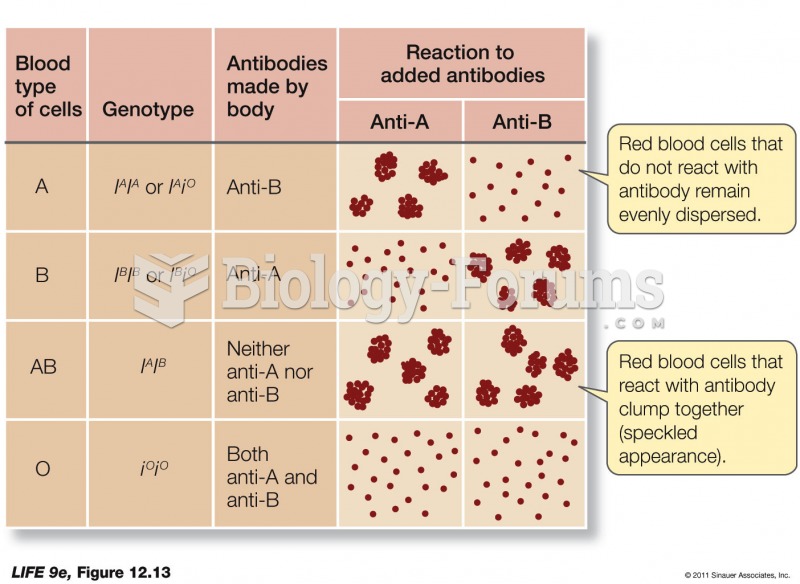
|
|
|
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
 Flower part number is a characteristic difference between monocots and eudicots (a) Flowers and buds
Flower part number is a characteristic difference between monocots and eudicots (a) Flowers and buds
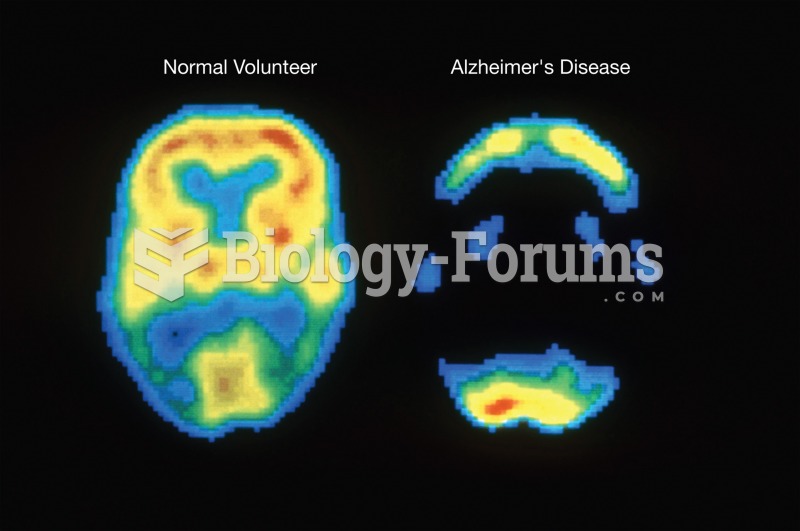 Positron emission tomography (PET) image showing the difference in the metabolic activity of the bra
Positron emission tomography (PET) image showing the difference in the metabolic activity of the bra