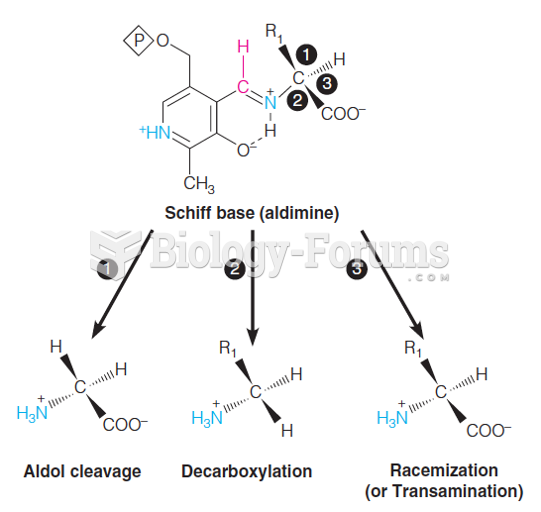
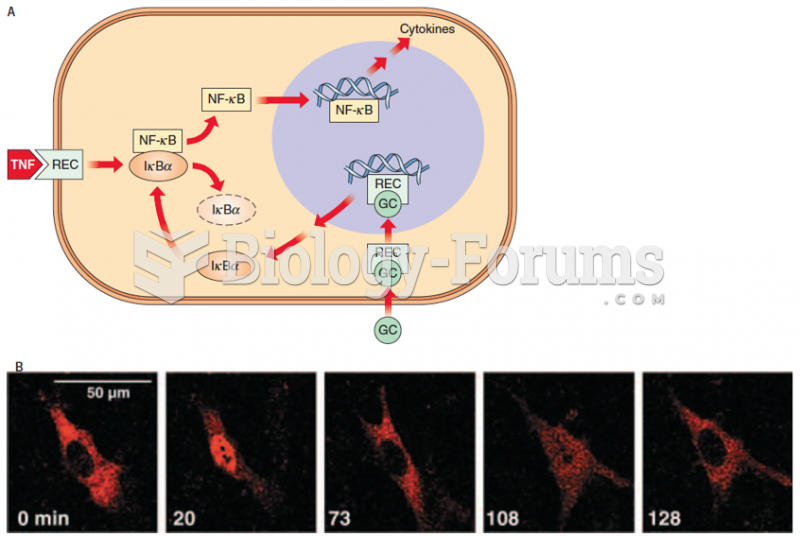
|
|
|
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
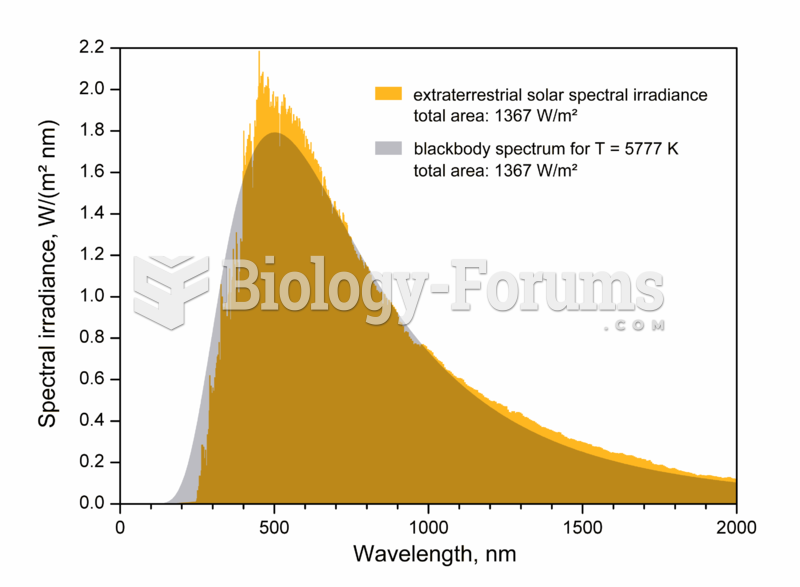 The effective temperature, or black body temperature, of the Sun (5777 K) is the temperature a black
The effective temperature, or black body temperature, of the Sun (5777 K) is the temperature a black
 Three days after the Boston Marathon explosion, the FBI released this photograph that had been taken
Three days after the Boston Marathon explosion, the FBI released this photograph that had been taken
 High rates of rural poverty have been a part of the United States from its origin to the present. ...
High rates of rural poverty have been a part of the United States from its origin to the present. ...