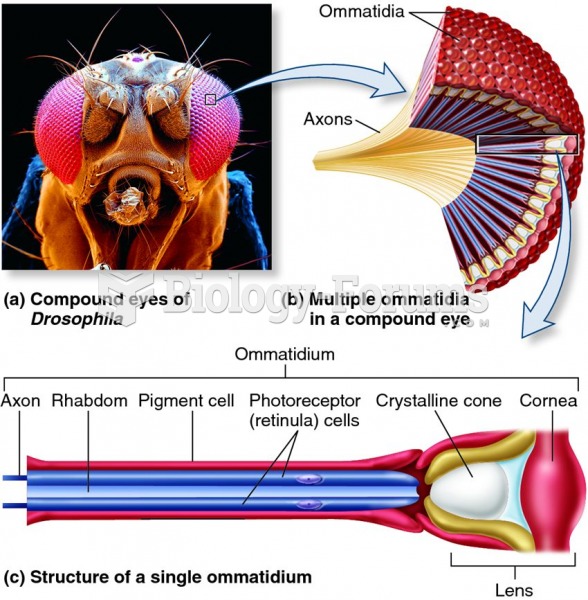
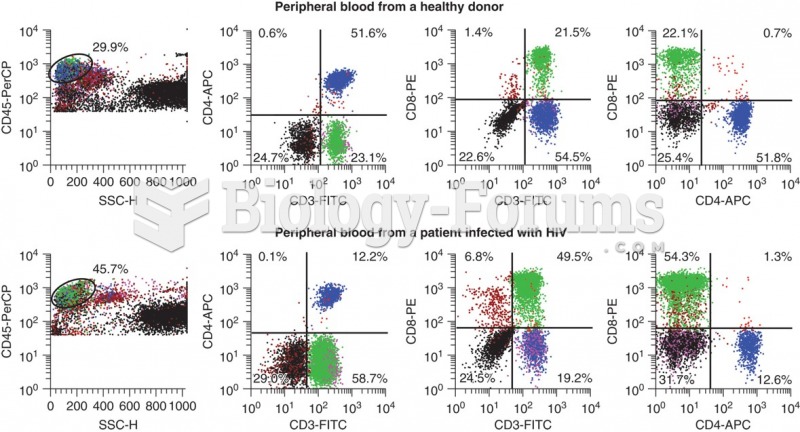
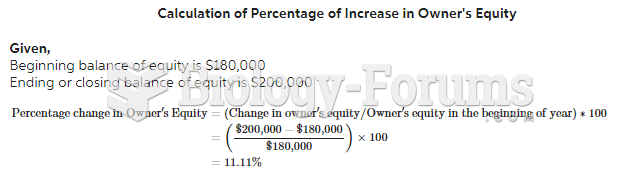
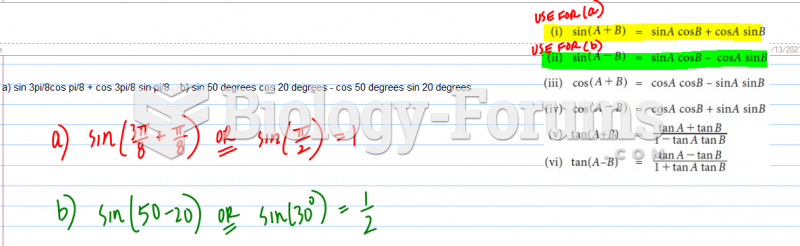|
|
|
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.
Cocaine was isolated in 1860 and first used as a local anesthetic in 1884. Its first clinical use was by Sigmund Freud to wean a patient from morphine addiction. The fictional character Sherlock Holmes was supposed to be addicted to cocaine by injection.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
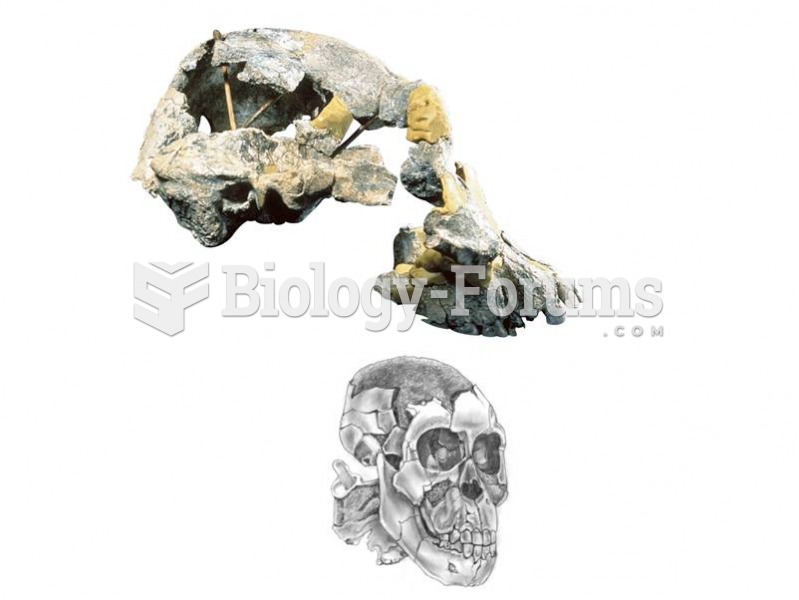 A complete cranium of Au. Afarensis from Hadar, Ethiopia, shows a prognathic face and a small brainc
A complete cranium of Au. Afarensis from Hadar, Ethiopia, shows a prognathic face and a small brainc
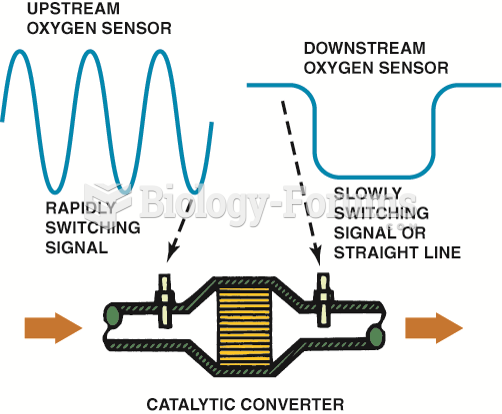 The OBD-II catalytic converter monitor compares the signals of the upstream and downstream O2Ss to ...
The OBD-II catalytic converter monitor compares the signals of the upstream and downstream O2Ss to ...