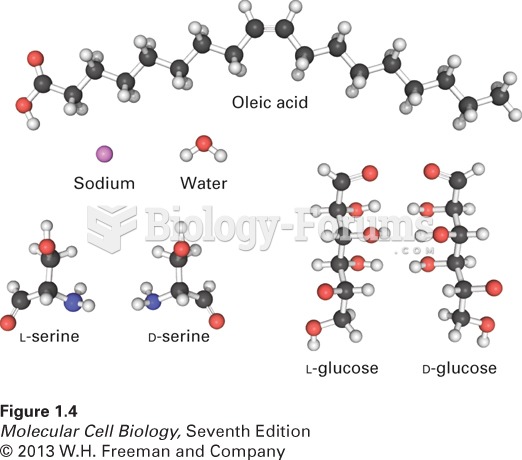
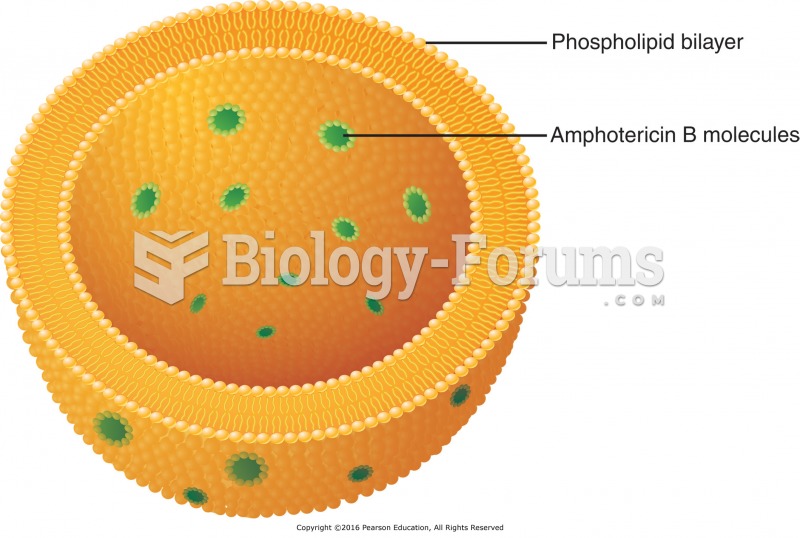
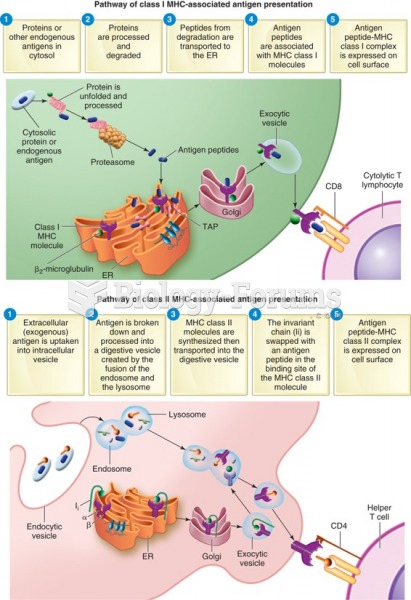
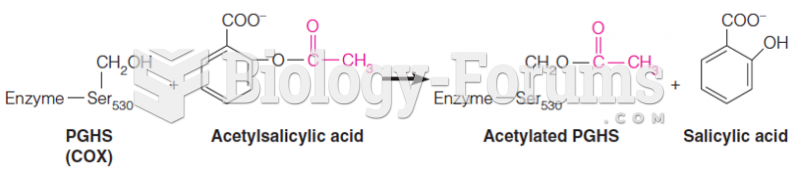
|
|
|
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Alzheimer's disease affects only about 10% of people older than 65 years of age. Most forms of decreased mental function and dementia are caused by disuse (letting the mind get lazy).
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.