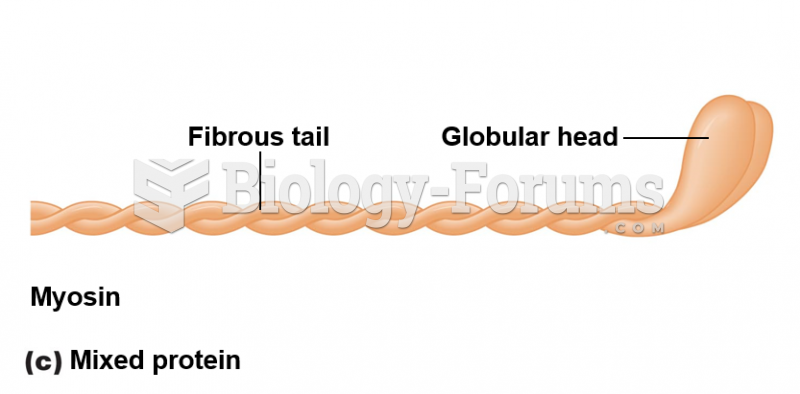
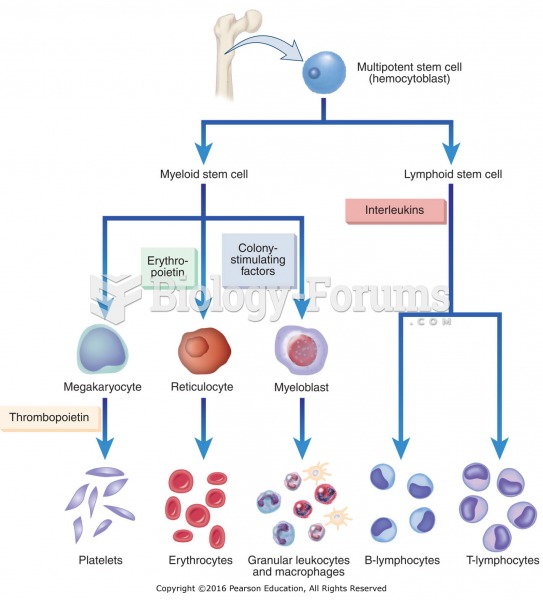
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
The senior population grows every year. Seniors older than 65 years of age now comprise more than 13% of the total population. However, women outlive men. In the 85-and-over age group, there are only 45 men to every 100 women.