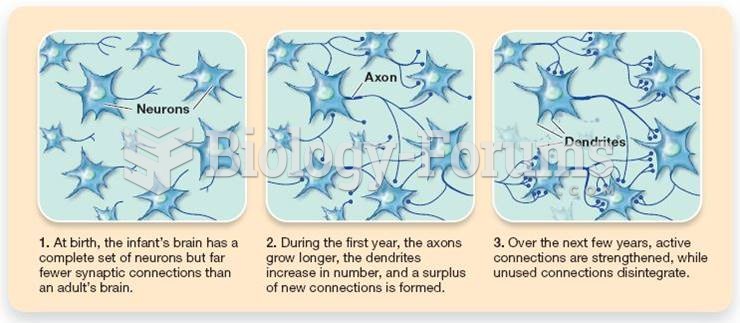
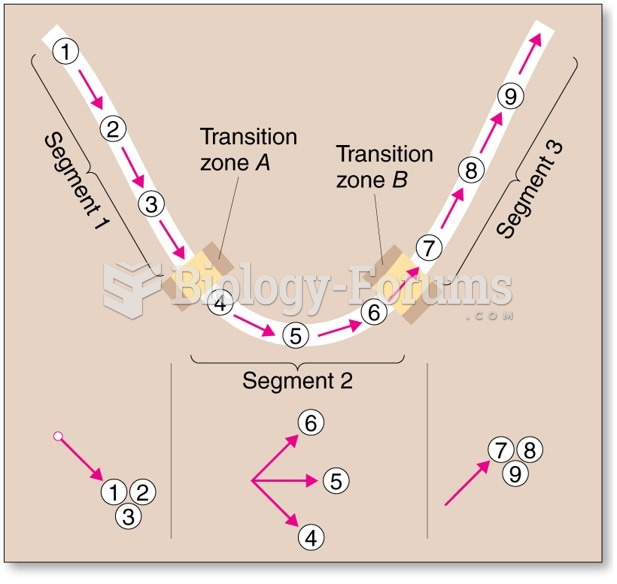
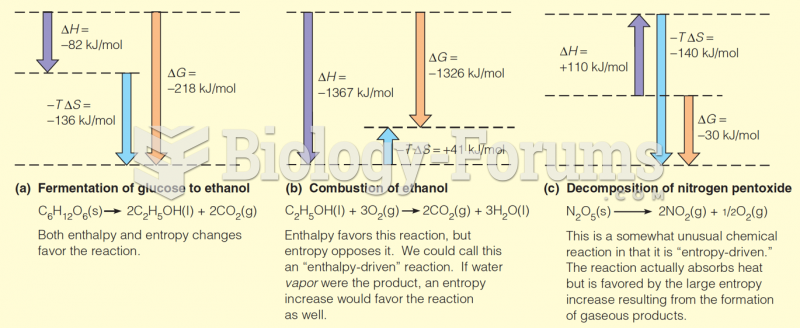
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Did you know?
When blood is deoxygenated and flowing back to the heart through the veins, it is dark reddish-blue in color. Blood in the arteries that is oxygenated and flowing out to the body is bright red. Whereas arterial blood comes out in spurts, venous blood flows.
Did you know?
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.