This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Did you know?
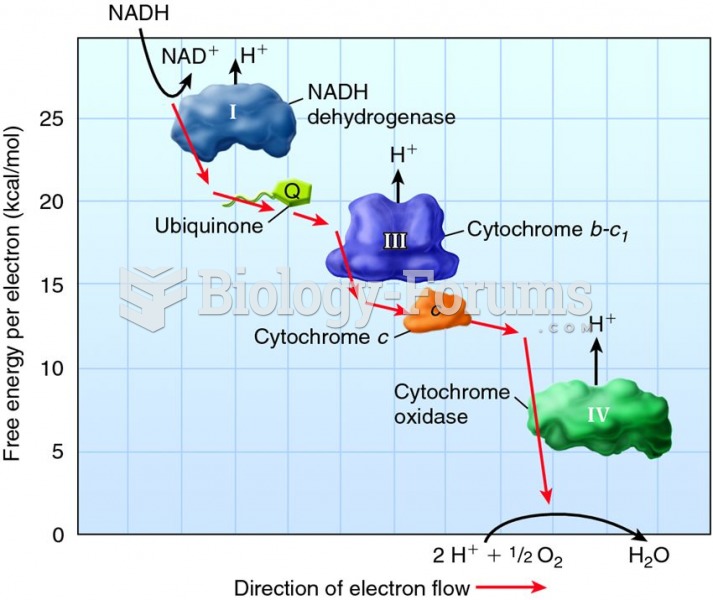
Cyanide works by making the human body unable to use oxygen.
Did you know?
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
Thyroid conditions may make getting pregnant impossible.