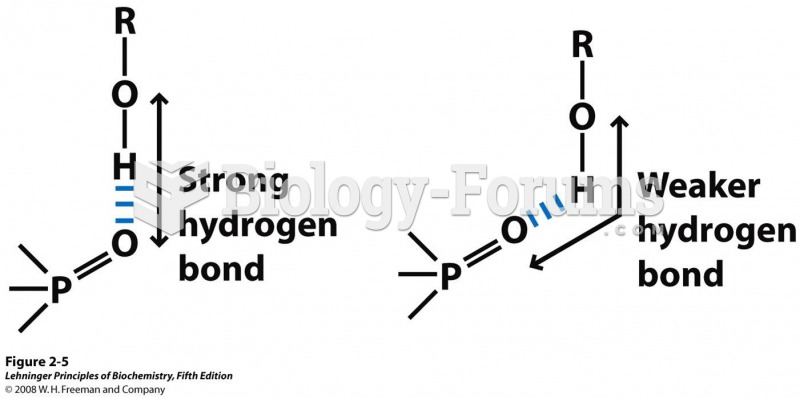
|
|
|
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Pope Sylvester II tried to introduce Arabic numbers into Europe between the years 999 and 1003, but their use did not catch on for a few more centuries, and Roman numerals continued to be the primary number system.
Medication errors are three times higher among children and infants than with adults.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.