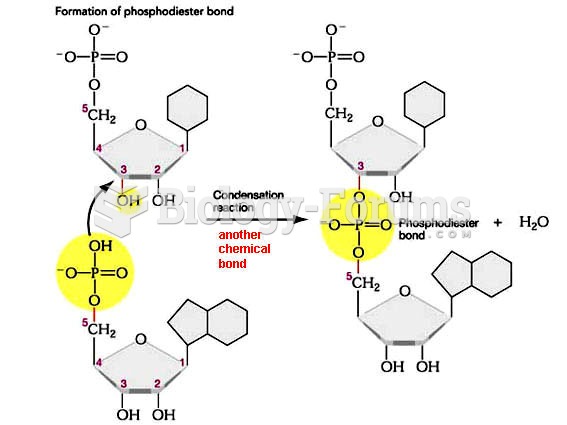
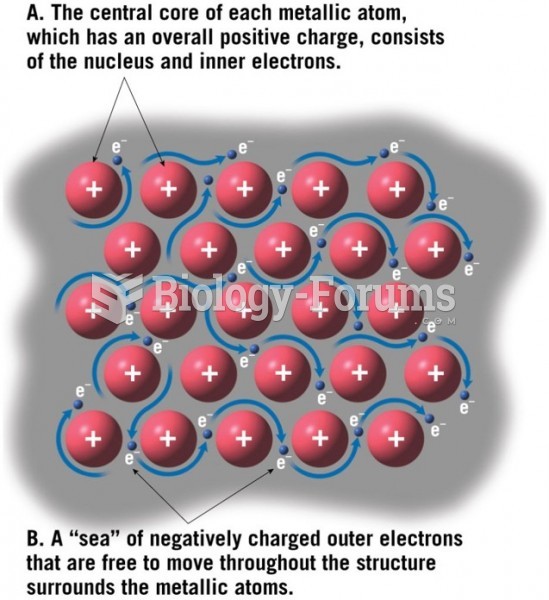
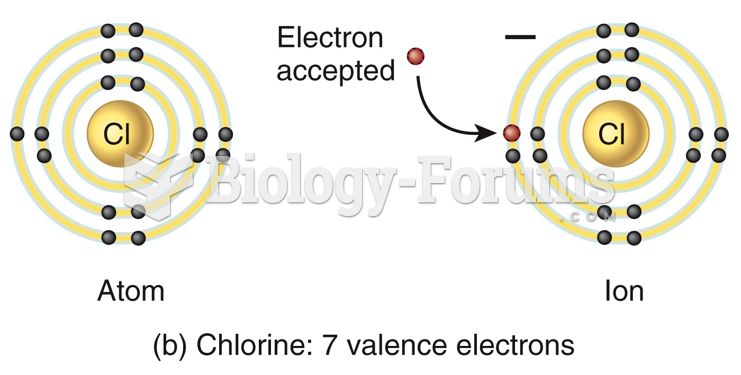
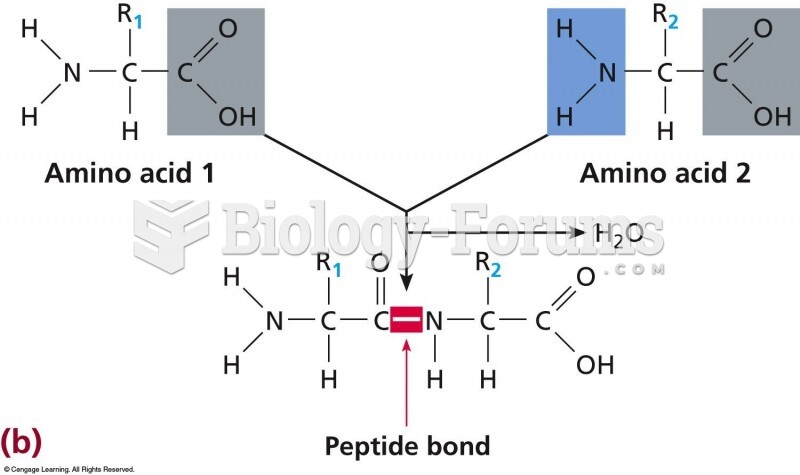
|
|
|
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
Approximately 25% of all reported medication errors result from some kind of name confusion.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.