|
|
|
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
Eating food that has been cooked with poppy seeds may cause you to fail a drug screening test, because the seeds contain enough opiate alkaloids to register as a positive.
 A container with gasoline containing alcohol. Notice the separation line where the alcohol-water ...
A container with gasoline containing alcohol. Notice the separation line where the alcohol-water ...
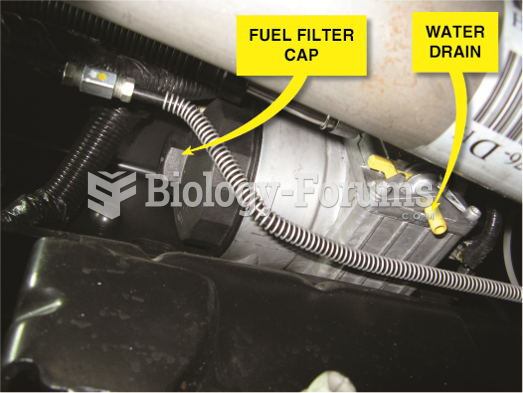 A fuel heater is part of the fuel filter and water separator located on the frame rail of a Ford ...
A fuel heater is part of the fuel filter and water separator located on the frame rail of a Ford ...