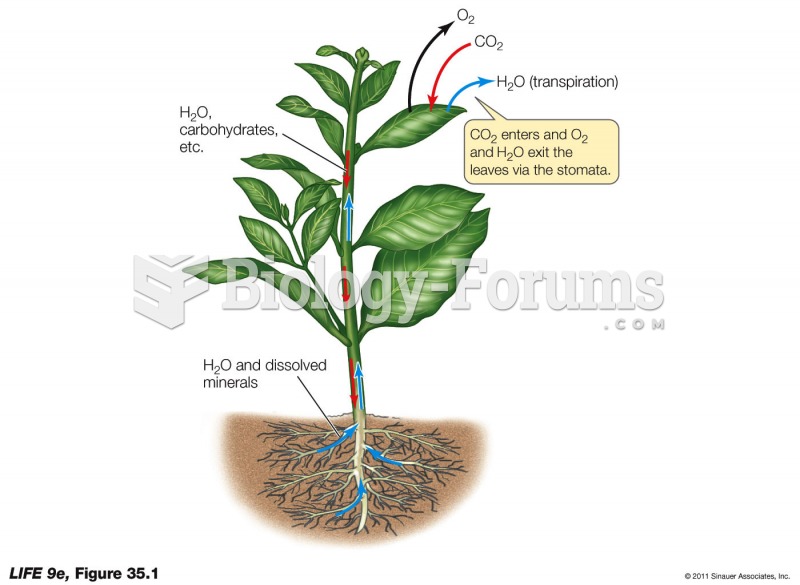
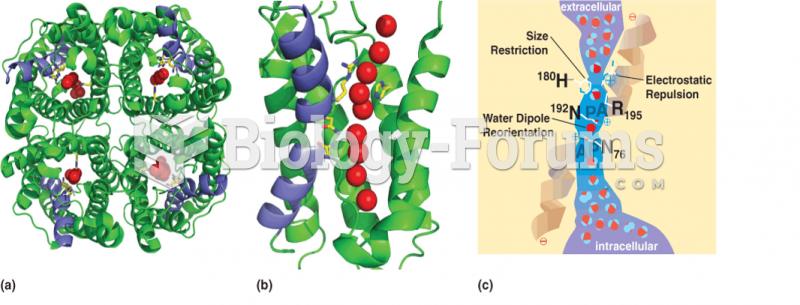
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
Did you know?
In 2010, opiate painkllers, such as morphine, OxyContin®, and Vicodin®, were tied to almost 60% of drug overdose deaths.
Did you know?
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.