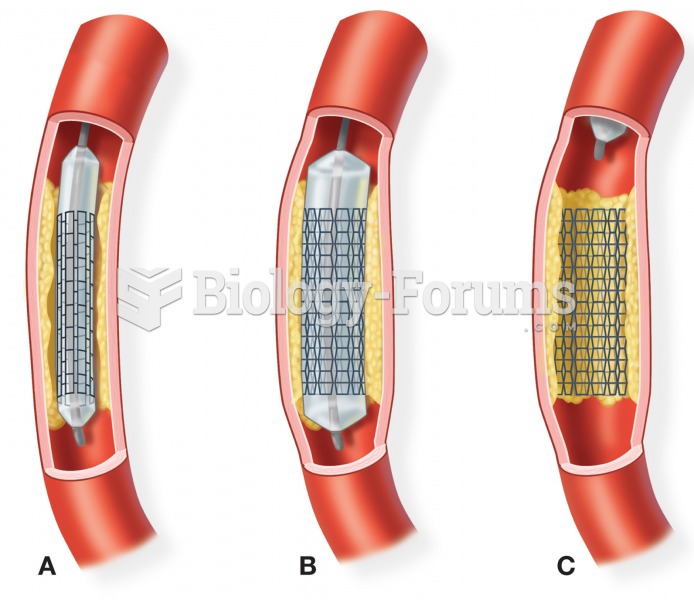
|
|
|
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
A cataract is a clouding of the eyes' natural lens. As we age, some clouding of the lens may occur. The first sign of a cataract is usually blurry vision. Although glasses and other visual aids may at first help a person with cataracts, surgery may become inevitable. Cataract surgery is very successful in restoring vision, and it is the most frequently performed surgery in the United States.
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
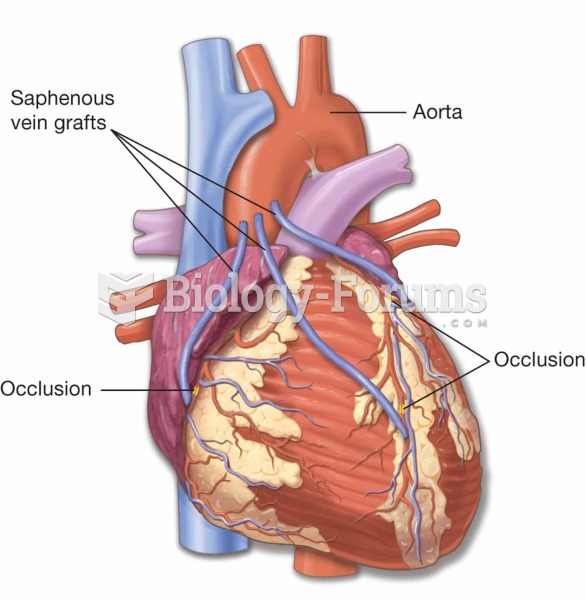 A coronary artery bypass graft (CABG) is a procedure to bypass a blocked coronary artery. The proced
A coronary artery bypass graft (CABG) is a procedure to bypass a blocked coronary artery. The proced
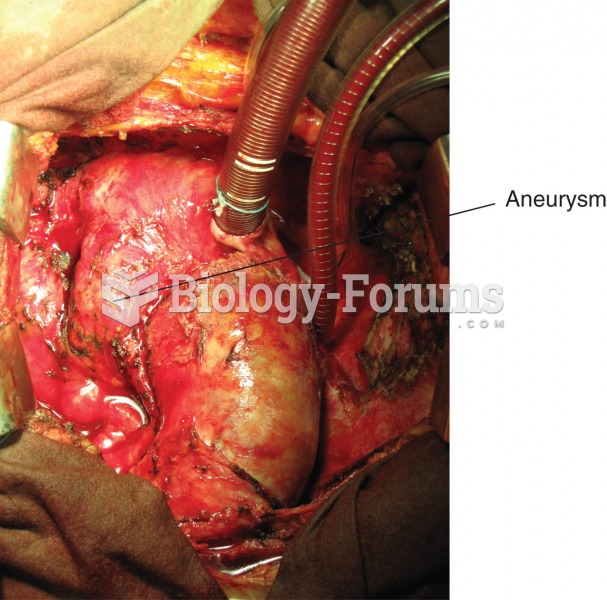 Aneurysm. Photograph of the aorta, the large blood vessel arising from the heart, with a large bulge
Aneurysm. Photograph of the aorta, the large blood vessel arising from the heart, with a large bulge
 Daniel Burnham’s Flatiron Building (1902) was one of the first to use steel girders to hold up the b
Daniel Burnham’s Flatiron Building (1902) was one of the first to use steel girders to hold up the b