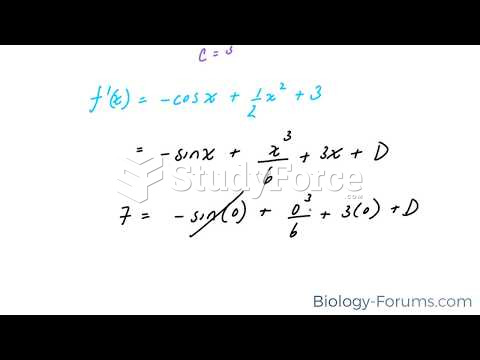This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
Did you know?
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
Did you know?
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.