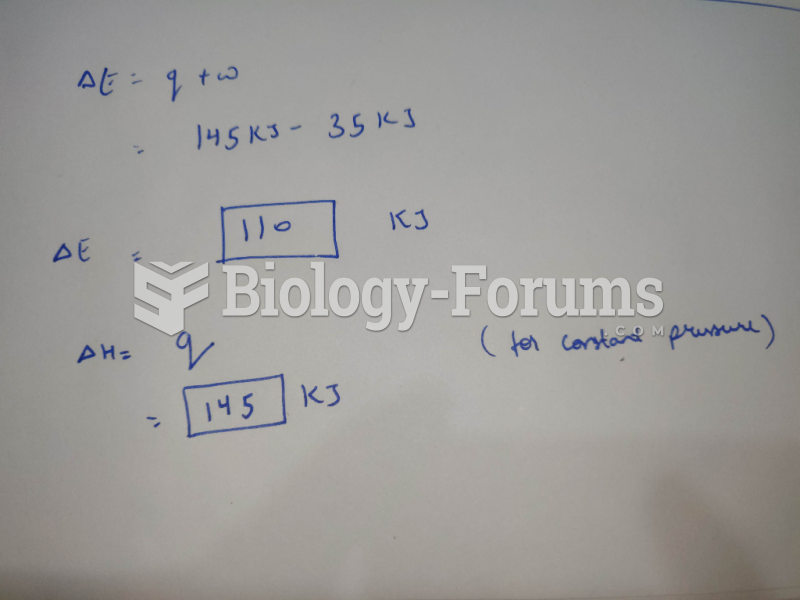
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.