This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Immunoglobulin injections may give short-term protection against, or reduce severity of certain diseases. They help people who have an inherited problem making their own antibodies, or those who are having certain types of cancer treatments.
Did you know?
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Did you know?
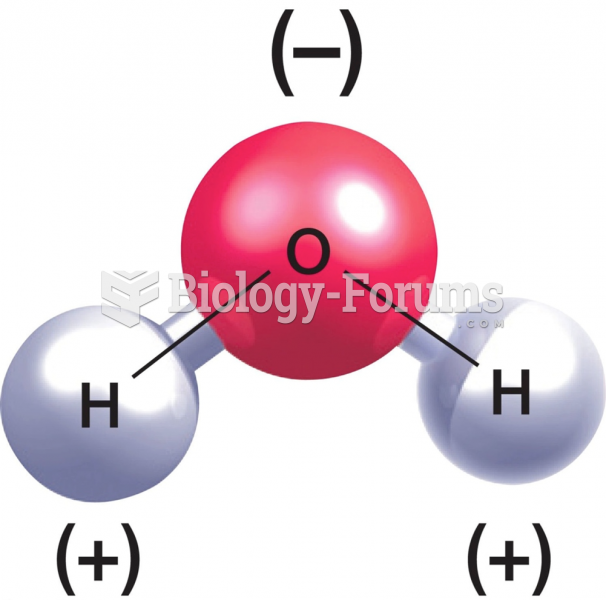
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
Inotropic therapy does not have a role in the treatment of most heart failure patients. These drugs can make patients feel and function better but usually do not lengthen the predicted length of their lives.