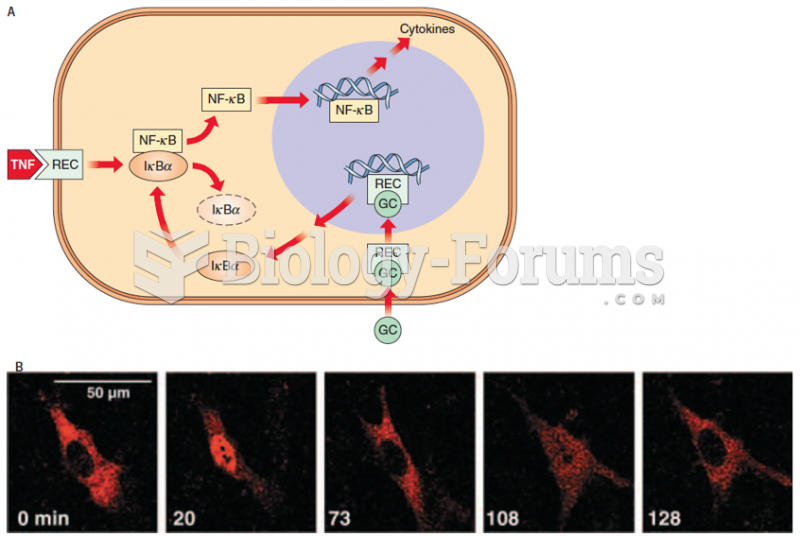
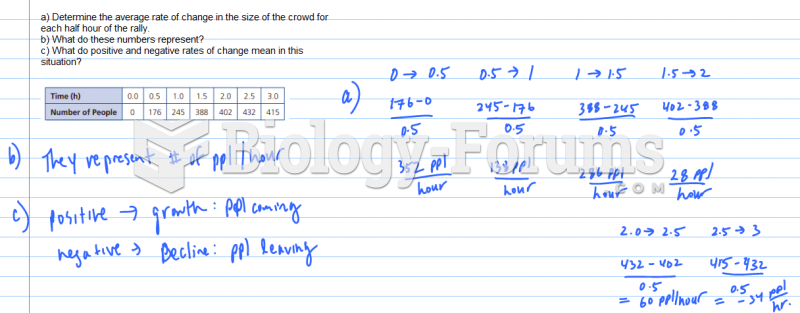
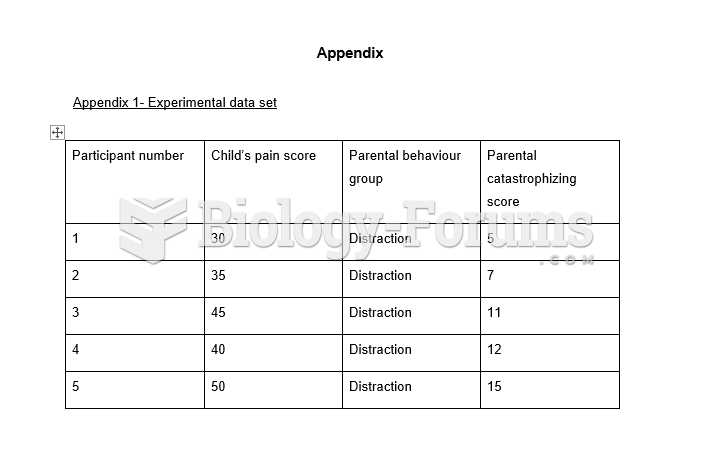
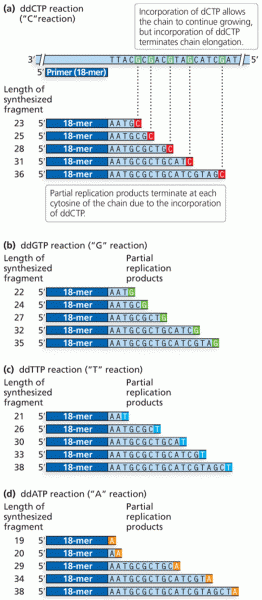
|
|
|
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Signs and symptoms that may signify an eye tumor include general blurred vision, bulging eye(s), double vision, a sensation of a foreign body in the eye(s), iris defects, limited ability to move the eyelid(s), limited ability to move the eye(s), pain or discomfort in or around the eyes or eyelids, red or pink eyes, white or cloud spots on the eye(s), colored spots on the eyelid(s), swelling around the eyes, swollen eyelid(s), and general vision loss.
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
 Temperament refers to stable differences in the emotional reactions of different children to similar ...
Temperament refers to stable differences in the emotional reactions of different children to similar ...
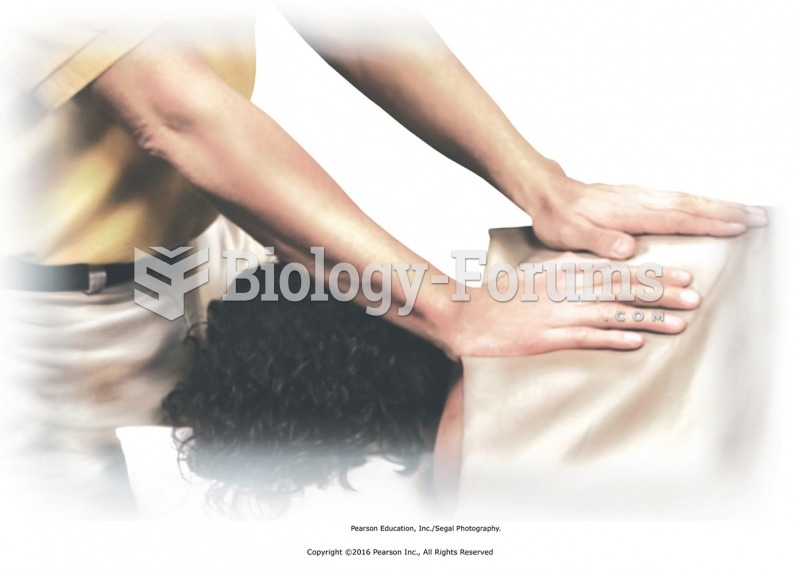 Redrape the back, face the head of the table, and apply passive touch as finishing technique. Gently ...
Redrape the back, face the head of the table, and apply passive touch as finishing technique. Gently ...