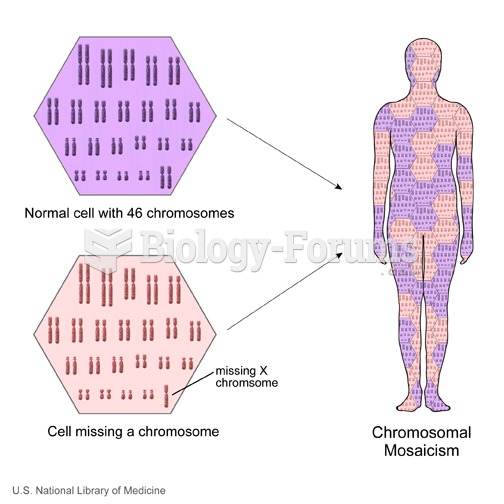
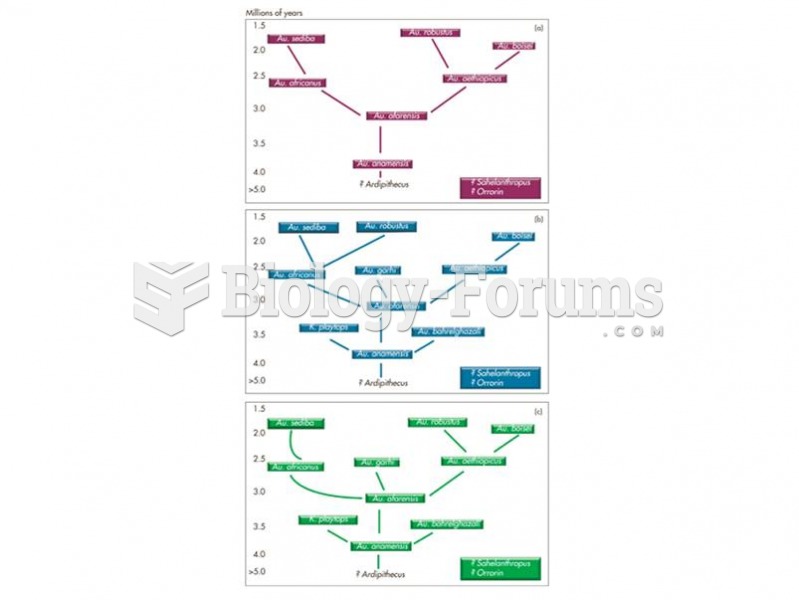
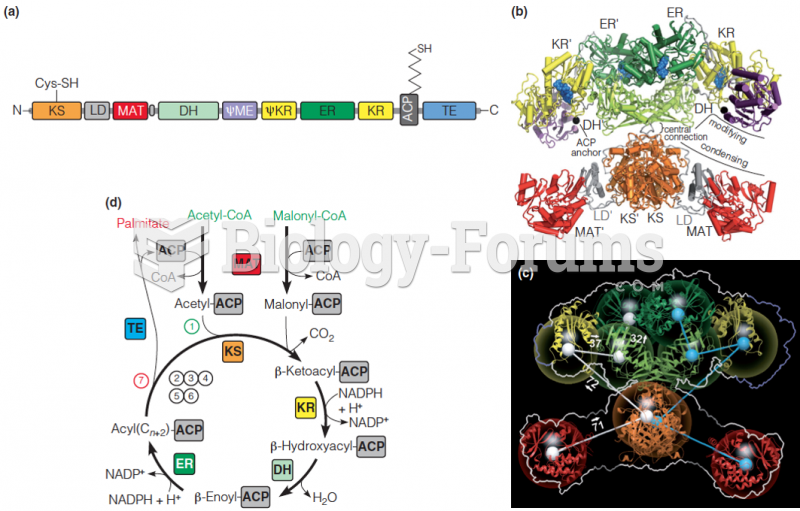
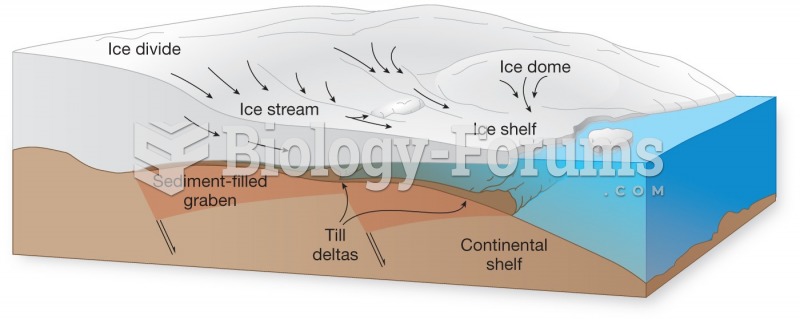
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
All adults should have their cholesterol levels checked once every 5 years. During 2009–2010, 69.4% of Americans age 20 and older reported having their cholesterol checked within the last five years.
Did you know?
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.