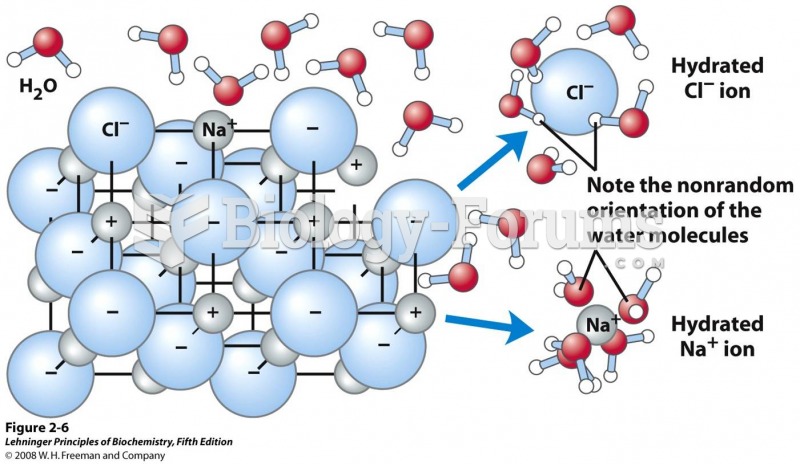
As u know solution is a homogeneous mixture composed of two substances solute and solvent. In such a mixture a solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature.
Solute,is the substance dissolved in fluid, forming a solution.In here, Solute glucose is dissolved in solvent water to get a Glucose solution
solubility or how much of solutes dissolve in a given volume of solvent depends on several factors and there is a maximum value for this at given conditions. this maximum also known as saturation is the point at which a solution of a substance can dissolve no more of that substance and additional amounts of it will appear as a precipitate
However, the point at which a solution can become saturated can change significantly with different environmental factors, such as temperature, pressure, and contamination. so u can change the solubility of Glucose by changing any of these factors.
but As i notice, u are asking abt ways to increase the rate of dissolution of solute. In this case Glucose . increase in solubility does not guarantee increase in dissolution rate,
A)but increase in temperature would increase the dissolution rate,
B)other than this stirring also would also increase the solubility rate .
C)use of well grounded glucose also would increase the rate
A)Increase temperature - Provides an increased amount of kinetic energy to the molecules allowing more of the molecules to have the necessary activation energy, Ea, to react. Therefore, the reaction speeds up.
Ex. An ice cube melts faster at 100°C than 50°C
In this case reaction is dissolution of Glucose in water
B,C)Increased surface area - Molecules can only react if they interact with one another (touch). By increasing the surface area, there will be more readily available molecules to react therefore, the reaction speeds up.
Ex. Powders dissolve faster than solids
Stirring would increase the interaction of molecules