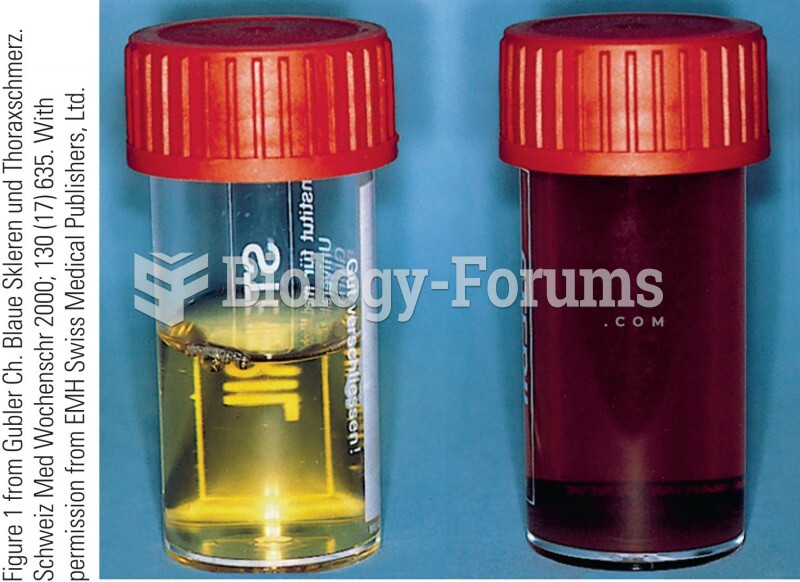
|
|
|
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
When Gabriel Fahrenheit invented the first mercury thermometer, he called "zero degrees" the lowest temperature he was able to attain with a mixture of ice and salt. For the upper point of his scale, he used 96°, which he measured as normal human body temperature (we know it to be 98.6° today because of more accurate thermometers).
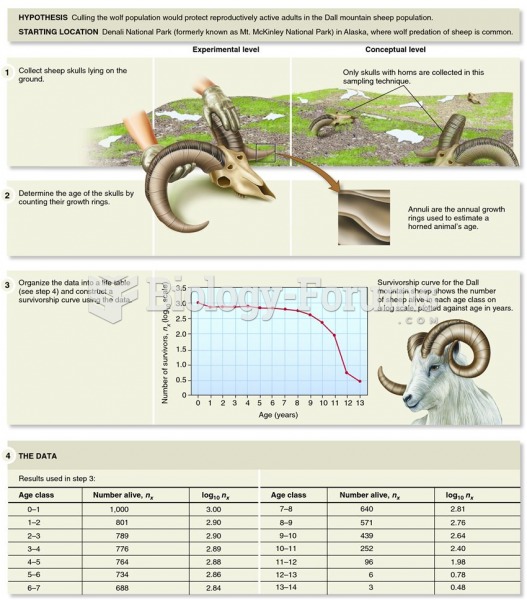 Examining the survivorship curve of a Dall mountain sheep population reveals information on the caus
Examining the survivorship curve of a Dall mountain sheep population reveals information on the caus
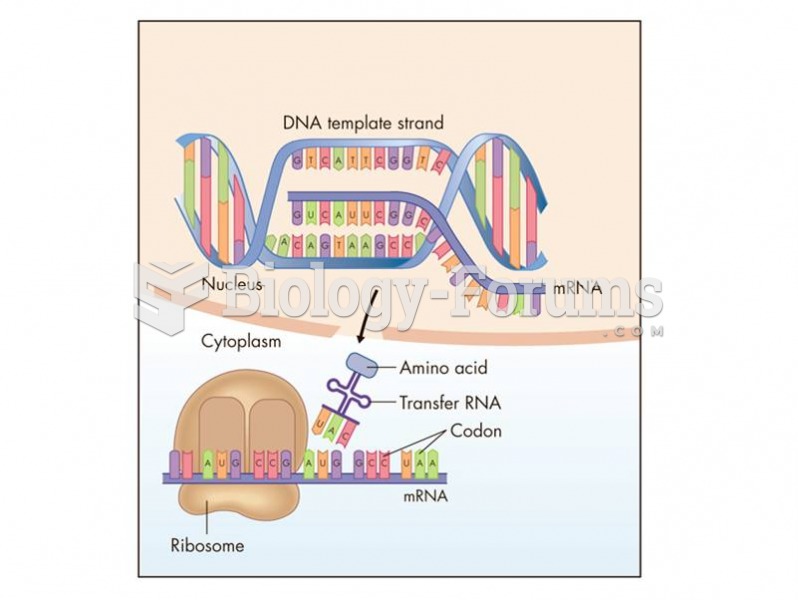 Messenger RNA (mRNA) carries genetic information from the nucleus to the cytoplasm for protein synth
Messenger RNA (mRNA) carries genetic information from the nucleus to the cytoplasm for protein synth