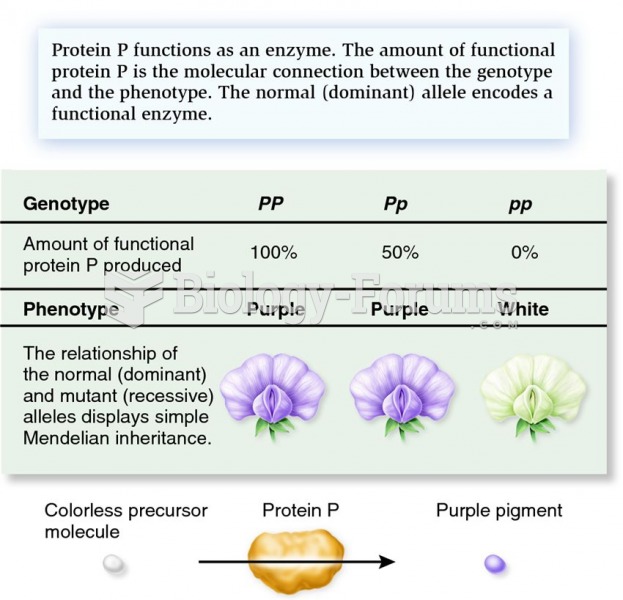
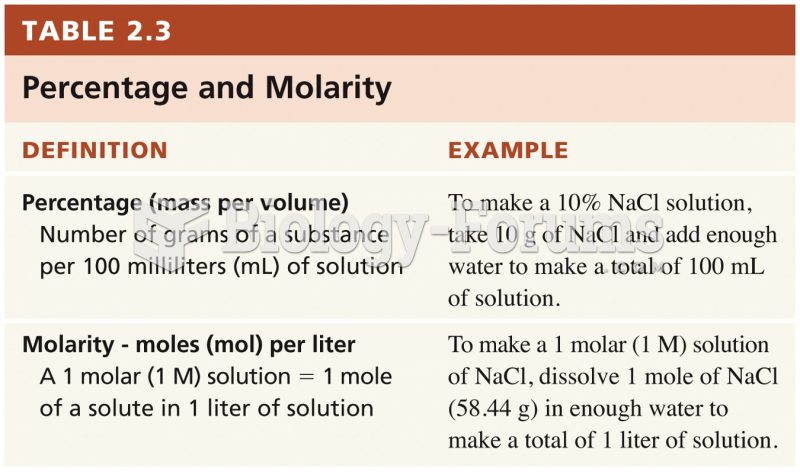
|
|
|
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
There are more bacteria in your mouth than there are people in the world.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Medication errors are more common among seriously ill patients than with those with minor conditions.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.