|
|
|
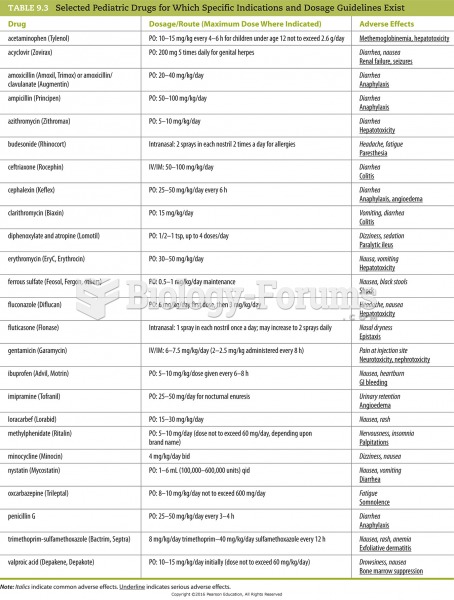
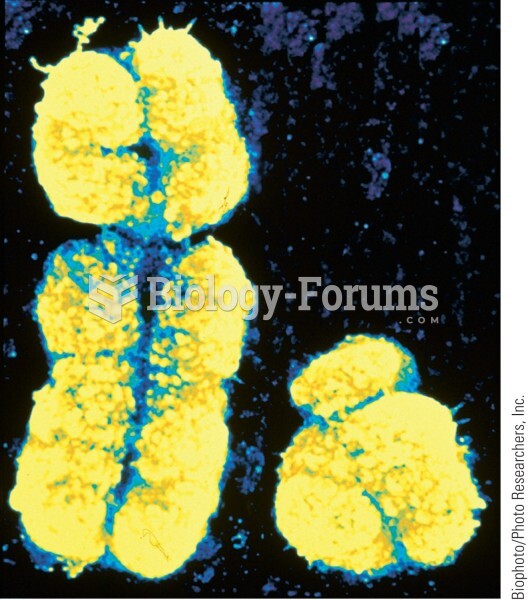
Disorders that may affect pharmacodynamics include genetic mutations, malnutrition, thyrotoxicosis, myasthenia gravis, Parkinson's disease, and certain forms of insulin-resistant diabetes mellitus.
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
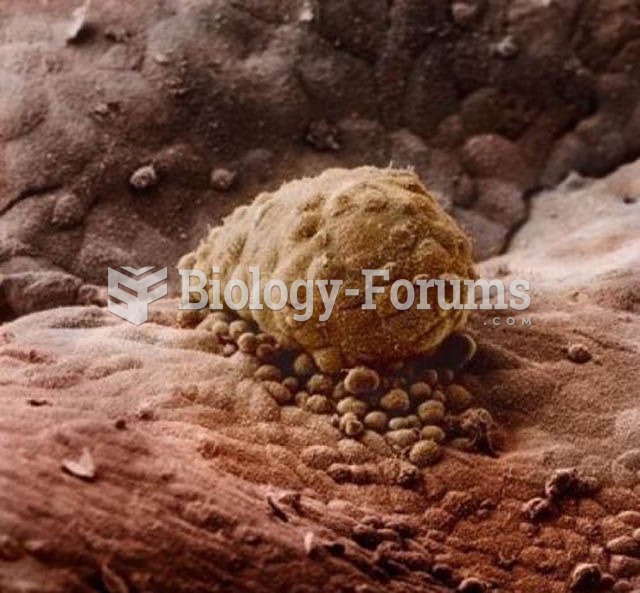
It is believed that humans initially contracted crabs from gorillas about 3 million years ago from either sleeping in gorilla nests or eating the apes.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
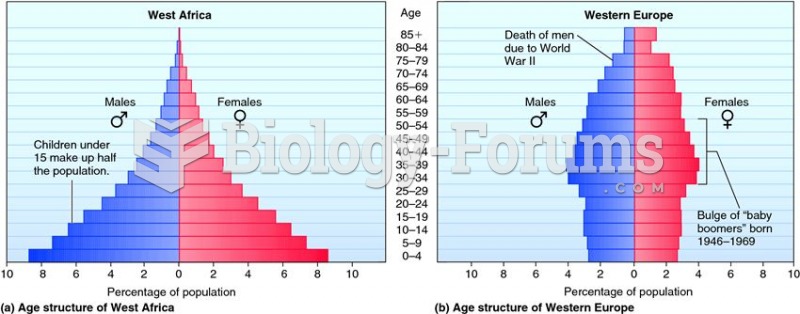
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.