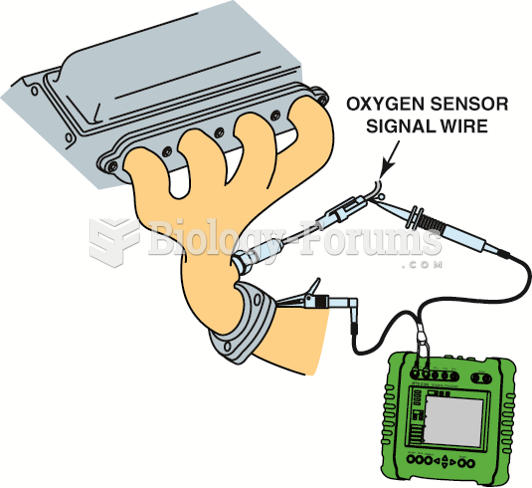
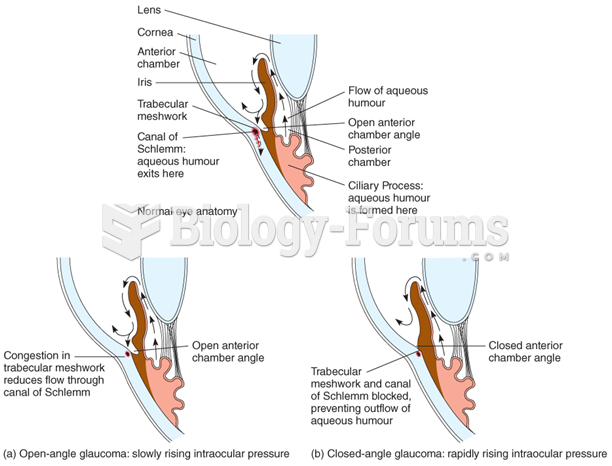
|
|
|
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
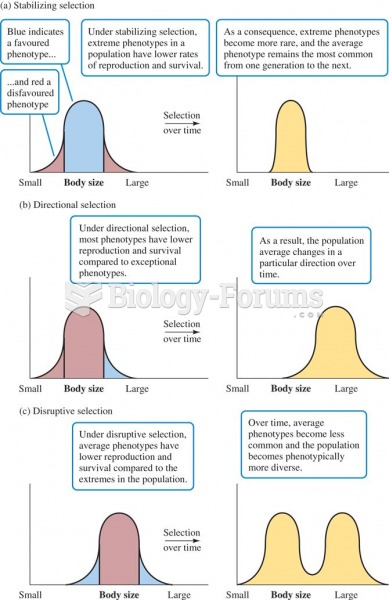 Three principle forms of natural selection: (a) stabilizing selection, (b) directional selection, an
Three principle forms of natural selection: (a) stabilizing selection, (b) directional selection, an
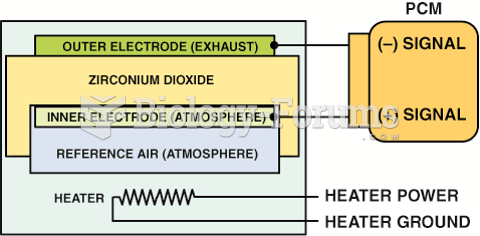 A planar design zirconia oxygen sensor places all of the elements together, which allows the sensor ...
A planar design zirconia oxygen sensor places all of the elements together, which allows the sensor ...