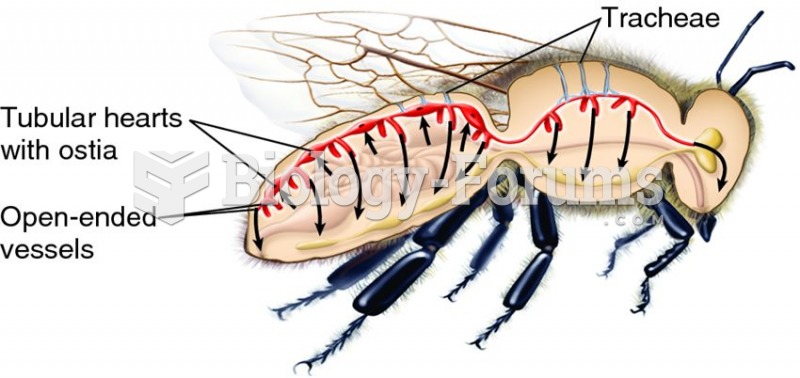
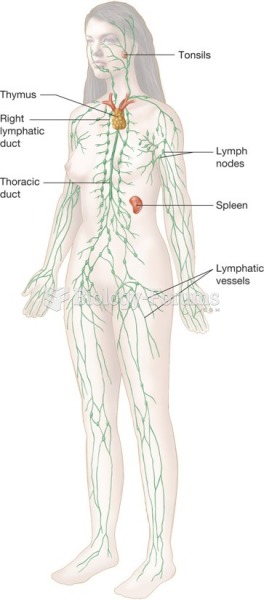
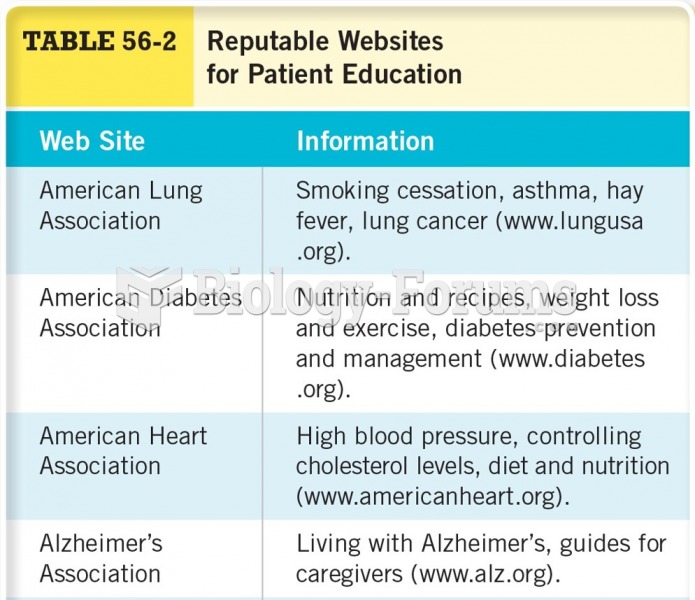
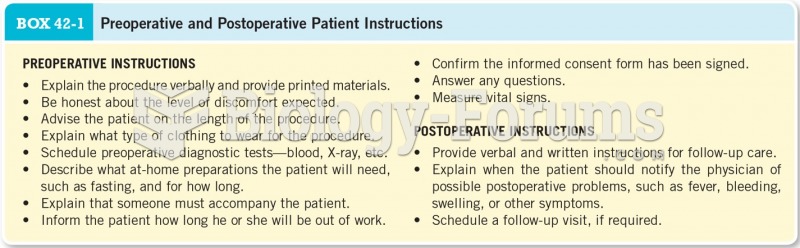
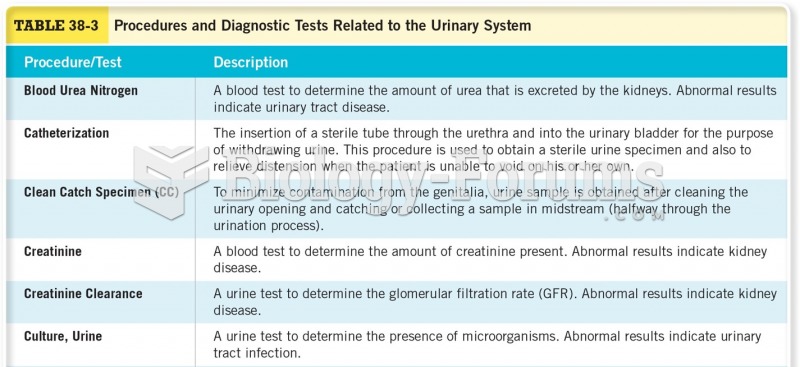
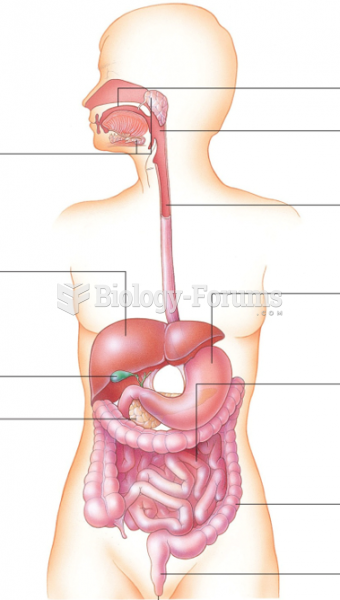
|
|
|
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
Fatal fungal infections may be able to resist newer antifungal drugs. Globally, fungal infections are often fatal due to the lack of access to multiple antifungals, which may be required to be utilized in combination. Single antifungals may not be enough to stop a fungal infection from causing the death of a patient.