|
|
|
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Ether was used widely for surgeries but became less popular because of its flammability and its tendency to cause vomiting. In England, it was quickly replaced by chloroform, but this agent caused many deaths and lost popularity.
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
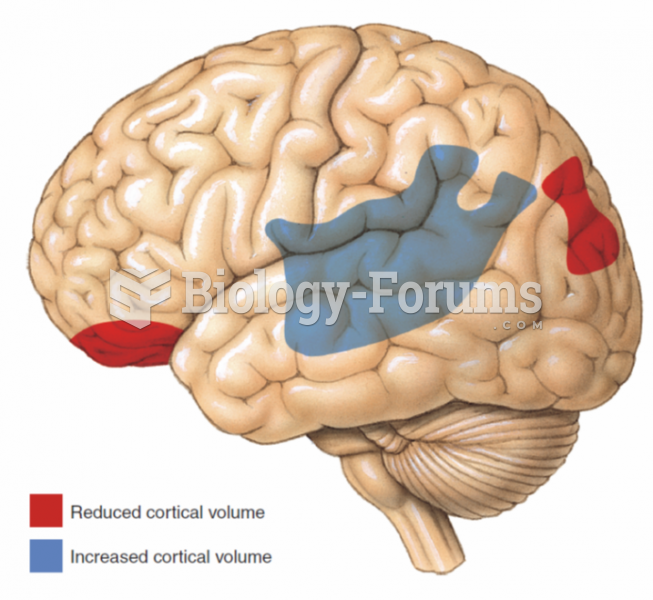
There are more nerve cells in one human brain than there are stars in the Milky Way.