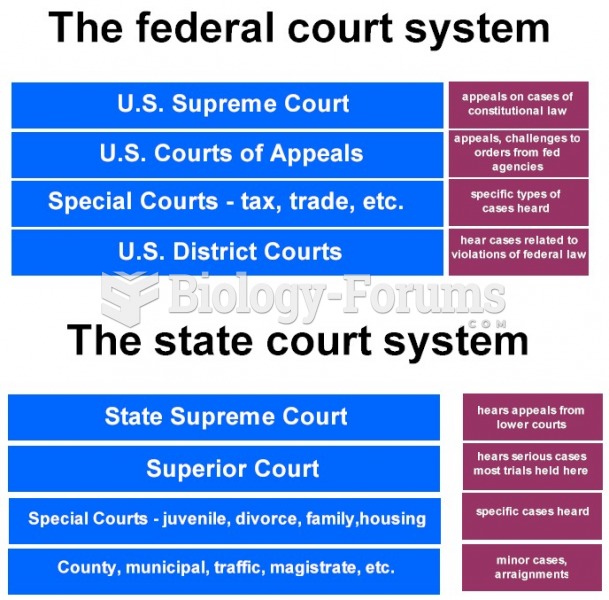
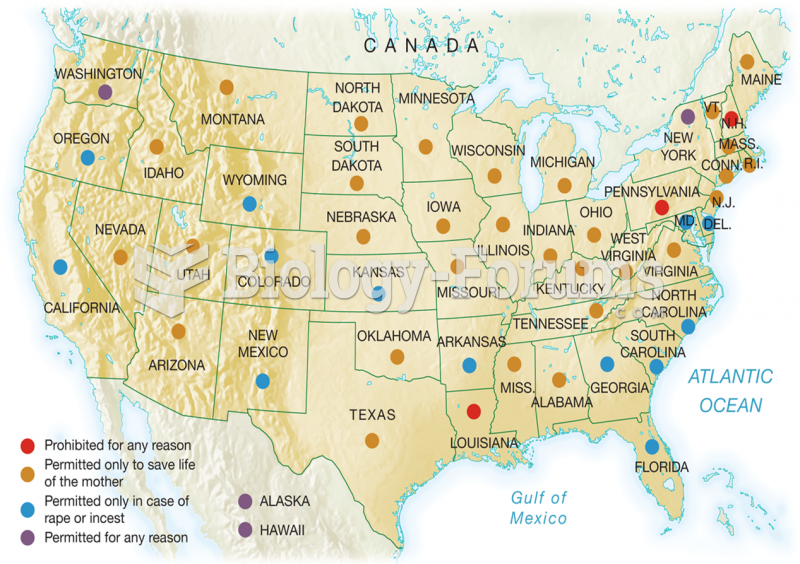
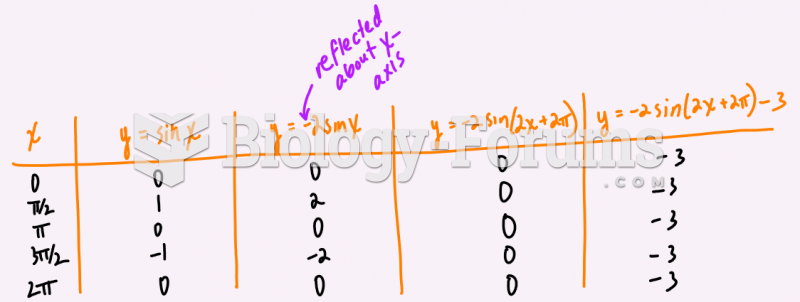
|
|
|
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
Carbamazepine can interfere with the results of home pregnancy tests. If you are taking carbamazepine, do not try to test for pregnancy at home.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.