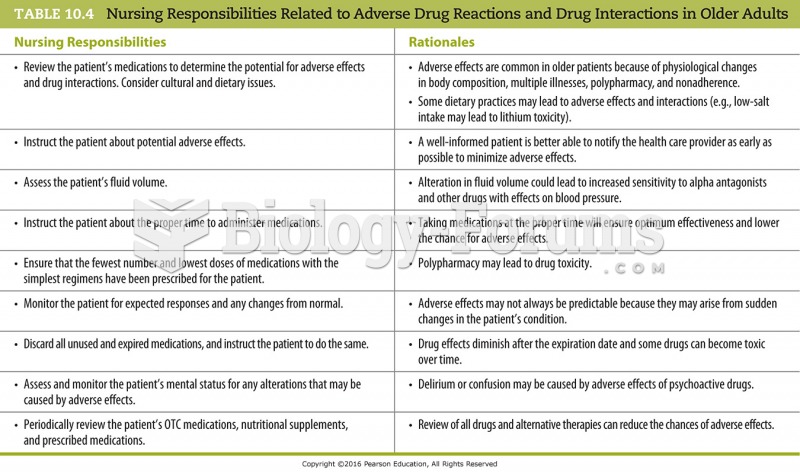
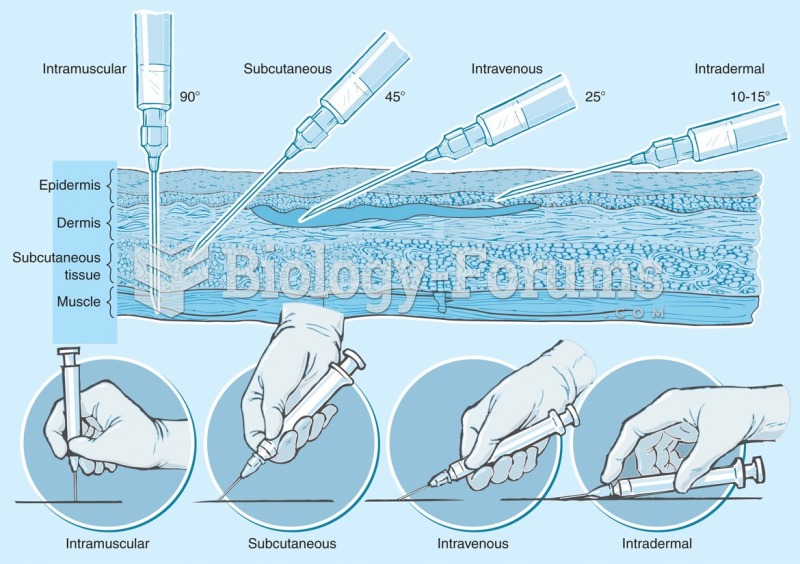
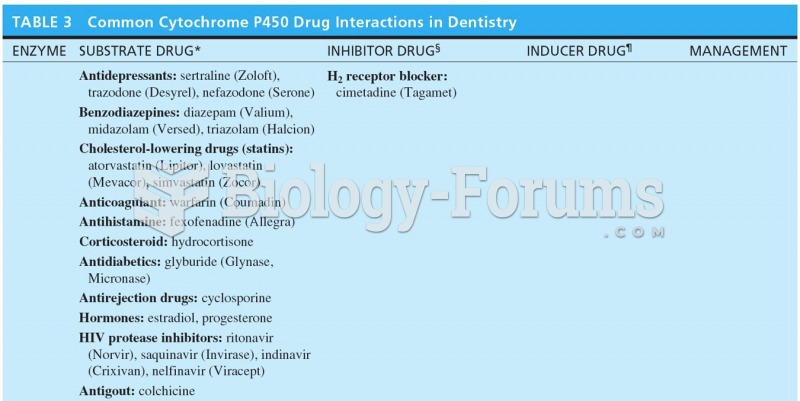
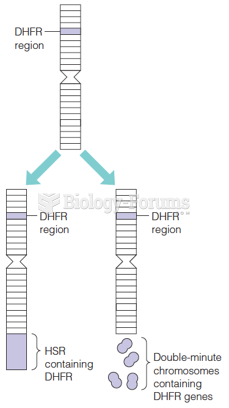
Answer to Question 1
3, 5, 1, 2, 4
Rationale: The U.S. Pharmacopoeia was established in 1820 and served as the first comprehensive publication of drug standards. The Biologics Control Act was passed in 1902 and controlled the quality of serums and other blood-related products. Passed in 1912, the Sherley Amendment made medicines safer by prohibiting the sale of drugs labeled with false therapeutic claims. The Childhood Vaccine Act was passed in 1986 and allowed the FDA to acquire information about clients taking vaccines, to recall biologics, and to recommend civil penalties if guidelines regarding biologic use were not followed. Lastly, in 1992 the Prescription Drug User Fee Act was passed requiring that nongeneric drug and biologic manufacturers pay fees to be used for improvements in the drug review process.
Answer to Question 2
1,3,4
Rationale 1: Most drugs do not proceed past the preclinical research stage of development because they are found to be either too toxic or just ineffective.
Rationale 2: Client variability and potential drug-to-drug interactions are examined in Phase 3 of the clinical investigation process after Food and Drug Administration (FDA) approval.
Rationale 3: The preclinical stage involves extensive testing on human, microbial cells, and animals to determine drug action and to predict whether the drug will cause harm to humans.
Rationale 4: Because lab tests cannot accurately predict human response to a drug, these results are always inconclusive.
Rationale 5: This extensive testing is done by the pharmaceutical company in the preclinical research stage of drug development, not the FDA.
Global Rationale: Most drugs do not proceed past the preclinical research stage of development because they are found to be either too toxic or just ineffective. Client variability and potential drug-to-drug interactions are examined in Phase 3 of the clinical investigation process after Food and Drug Administration (FDA) approval. The preclinical stage involves extensive testing on human, microbial cells, and animals to determine drug action and to predict whether the drug will cause harm to humans. Because lab tests cannot accurately predict human response to a drug, these results are always inconclusive. This extensive testing is done by the pharmaceutical company in the preclinical research stage of drug development, not the FDA.