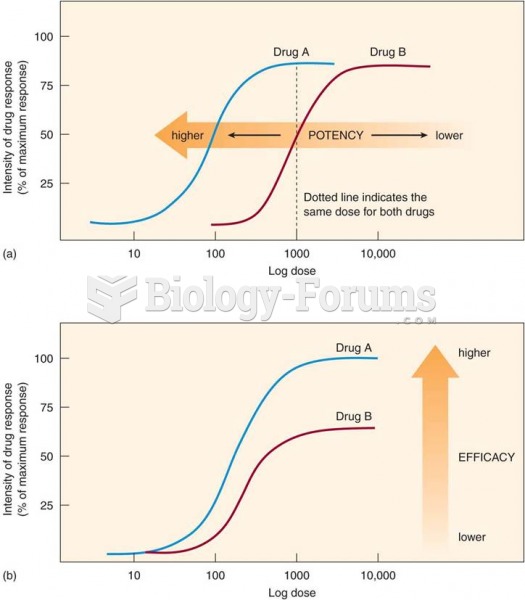
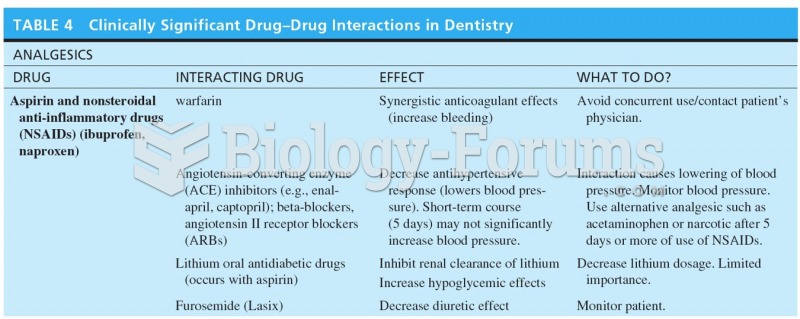
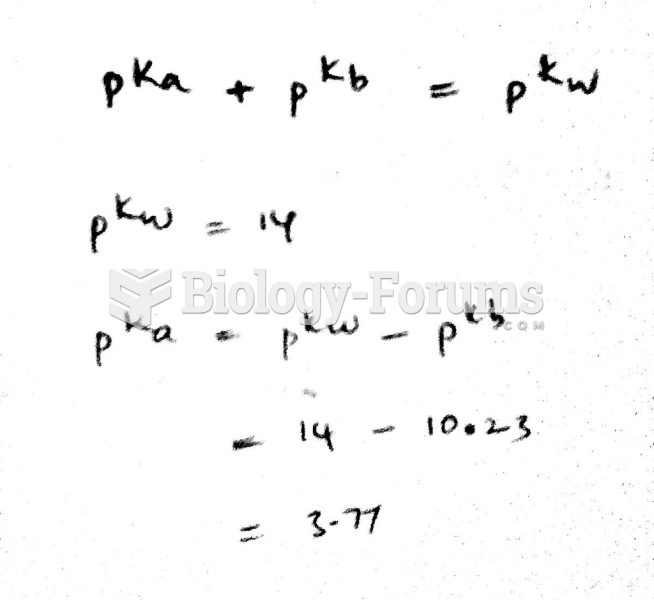Answer to Question 1
Correct Answer: 1,2,3
Rationale 1: The strength and purity of the products varied considerably because they were dependent on the experience of the pharmacist and the quality of the local ingredients, which could vary from region to region and batch to batch.
Rationale 2: The strength and purity of the products varied considerably because they were dependent on the experience of the pharmacist and the quality of the local ingredients, which could vary from region to region and batch to batch.
Rationale 3: The strength and purity of the products varied considerably because they were dependent on the experience of the pharmacist and the quality of the local ingredients, which could vary from region to region and batch to batch.
Rationale 4: Strength and purity could not be guaranteed, even if produced locally. Causing a hardship on those outside the region had nothing to do with determining that standardization was needed.
Rationale 5: Extensive testing prior to marketing did not occur until the early 1930s.
Global Rationale: The strength and purity of the products varied considerably because they were dependent on the experience of the pharmacist and the quality of the local ingredients, which could vary from region to region and batch to batch. Because of this, standardization was necessary. Strength and purity could not be guaranteed, even if produced locally. Causing a hardship on those outside the region had nothing to do with determining that standardization was needed. Extensive testing prior to marketing did not occur until the early 1930s.
Answer to Question 2
Correct Answer: 1,2
Rationale 1: The fact that manufacturers did not have to prove efficacy was a tremendous weakness in the regulation of drugs in the early 20th century.
Rationale 2: The PFDA of 1906 did not address false therapeutic claims.
Rationale 3: Requiring drug labels to identify their contents is not a weakness of the PFDA.
Rationale 4: The PFDA did not require testing for safety prior to marketing. It was not until Congress passed the Food, Drug, and Cosmetic Act that drugs had to be tested for safety prior to marketing.
Rationale 5: The act that encouraged the research and development of drugs for rare or unusual disorders is called the Orphan Act.
Global Rationale: The weaknesses of the PFDA of 1906 include the fact that manufacturers did not have to prove efficacy in the regulation of drugs in the early 20th century and the Act did not address false therapeutic claims. Requiring drug labels to identify their contents is not a weakness of the PFDA. The PFDA did not require testing for safety prior to marketing. It was not until Congress passed the Food, Drug, and Cosmetic Act that drugs had to be tested for safety prior to marketing. The act that encouraged the research and development of drugs for rare or unusual disorders is called the Orphan Act.