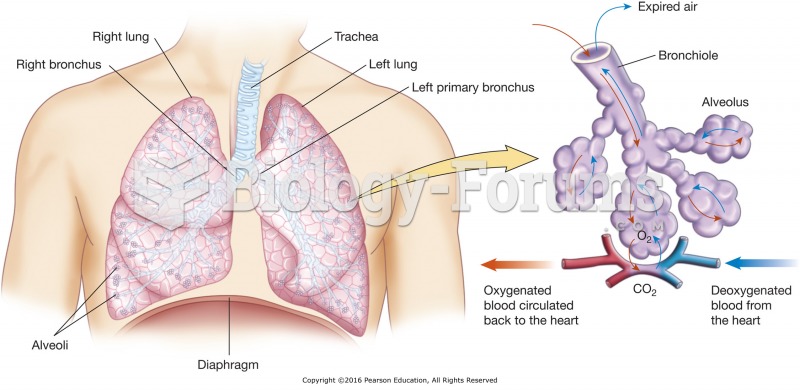
|
|
|
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
There are 20 feet of blood vessels in each square inch of human skin.
When intravenous medications are involved in adverse drug events, their harmful effects may occur more rapidly, and be more severe than errors with oral medications. This is due to the direct administration into the bloodstream.