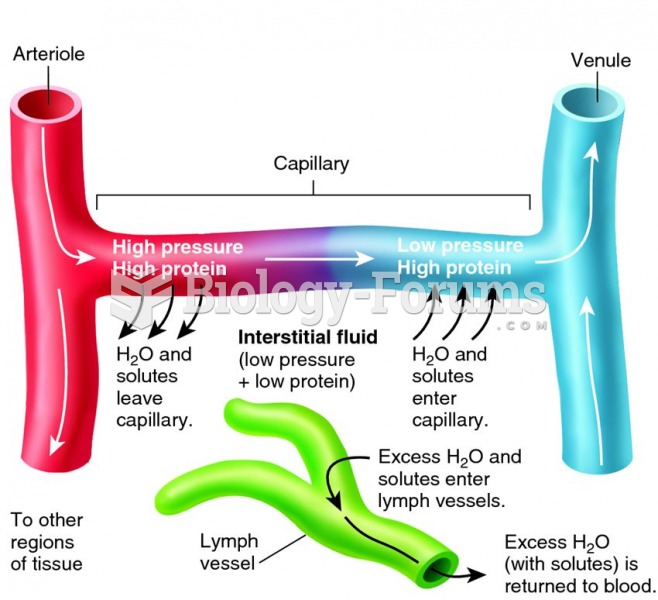
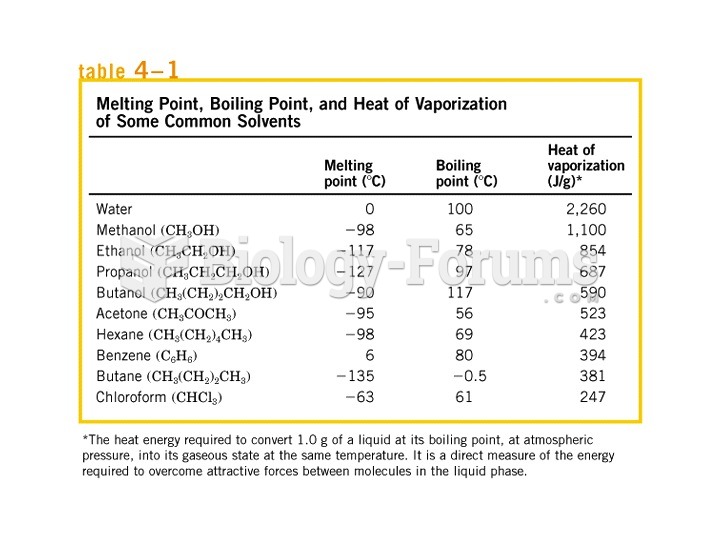
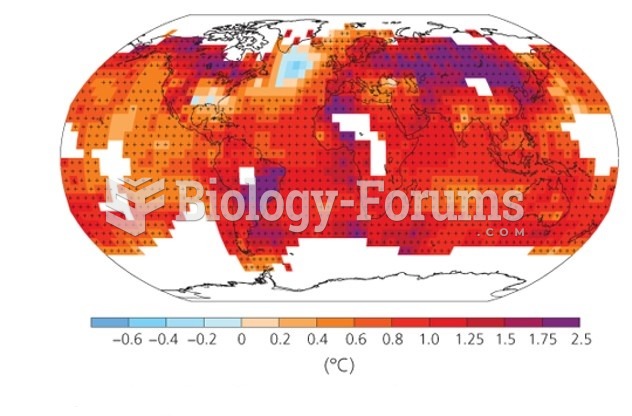
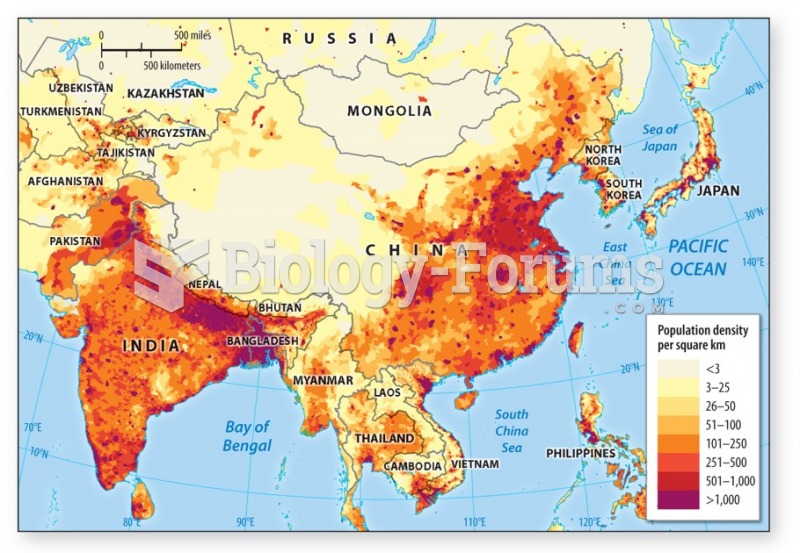
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.