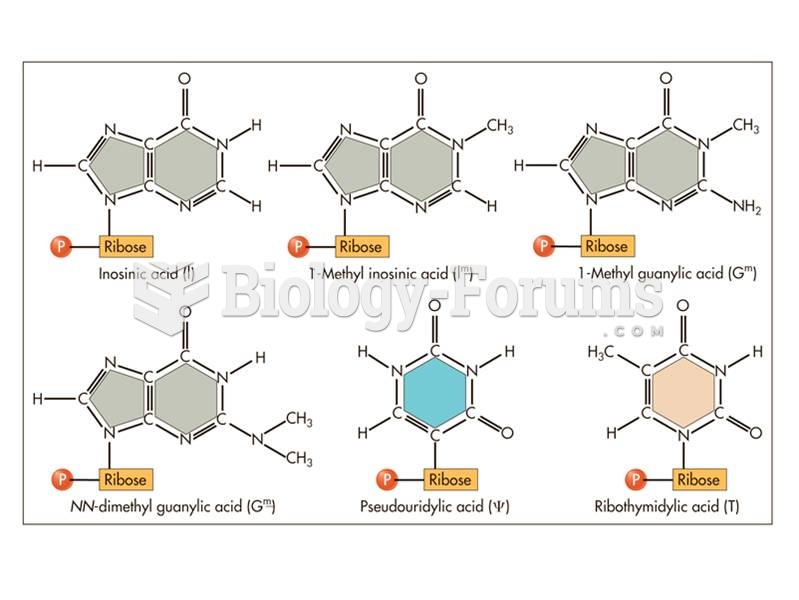
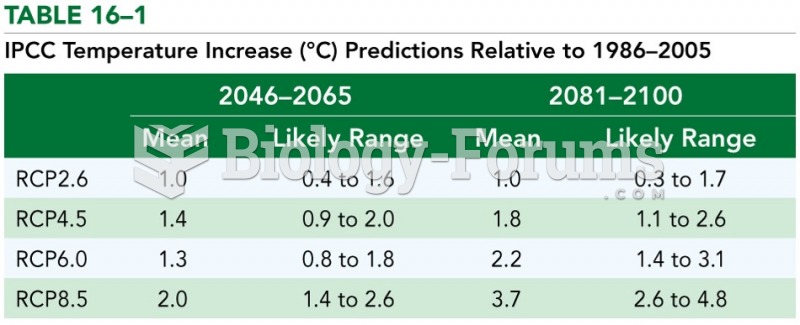
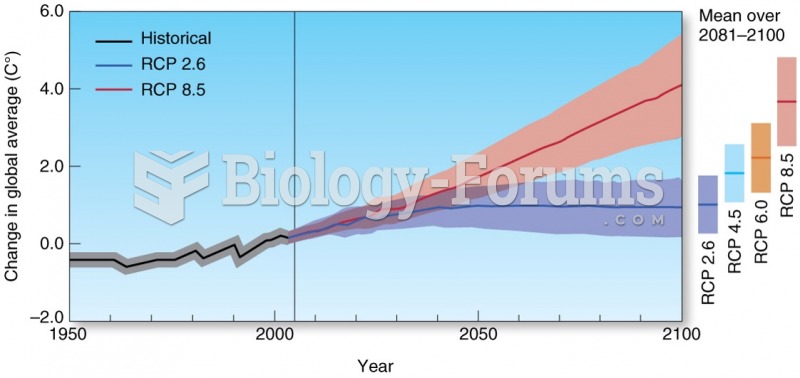
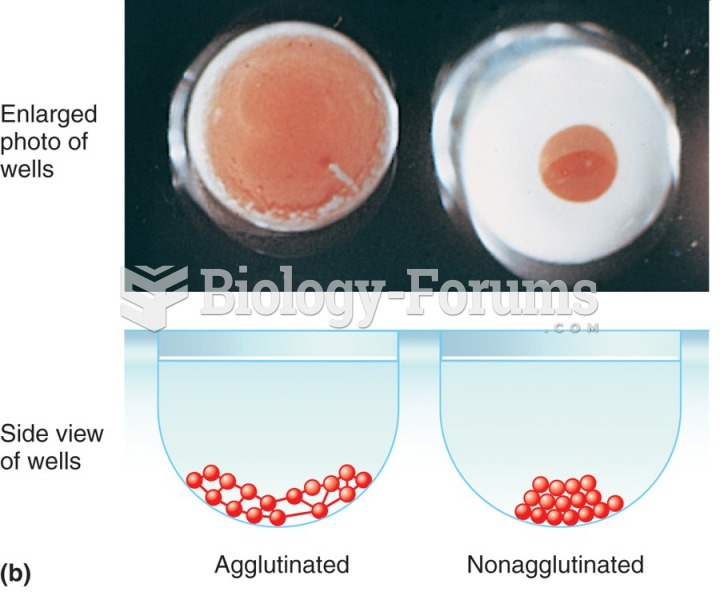
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
Anti-aging claims should not ever be believed. There is no supplement, medication, or any other substance that has been proven to slow or stop the aging process.
Did you know?
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.