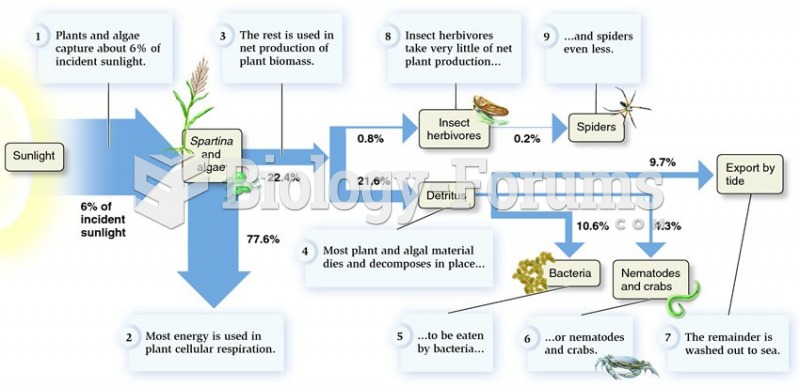
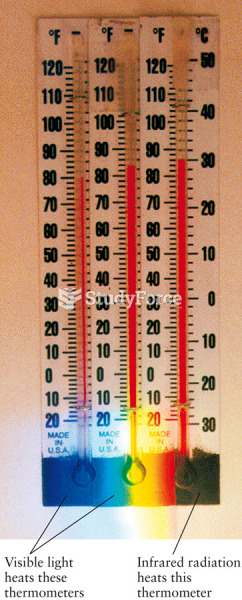
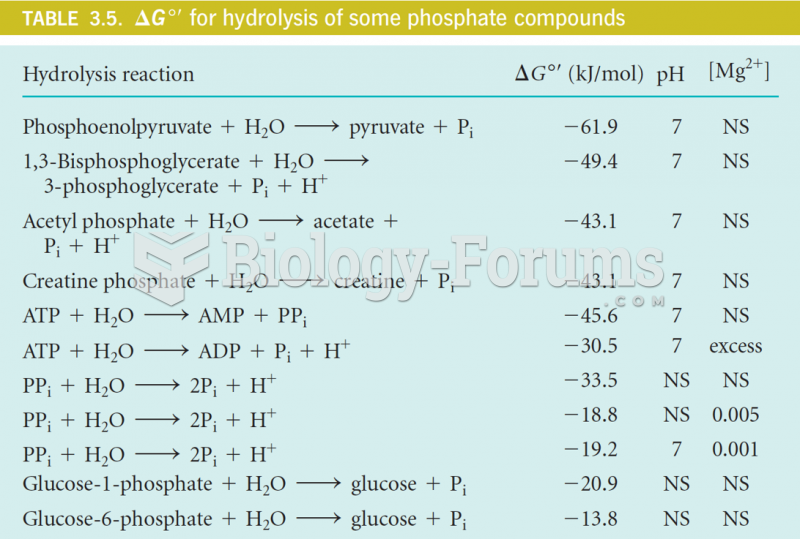
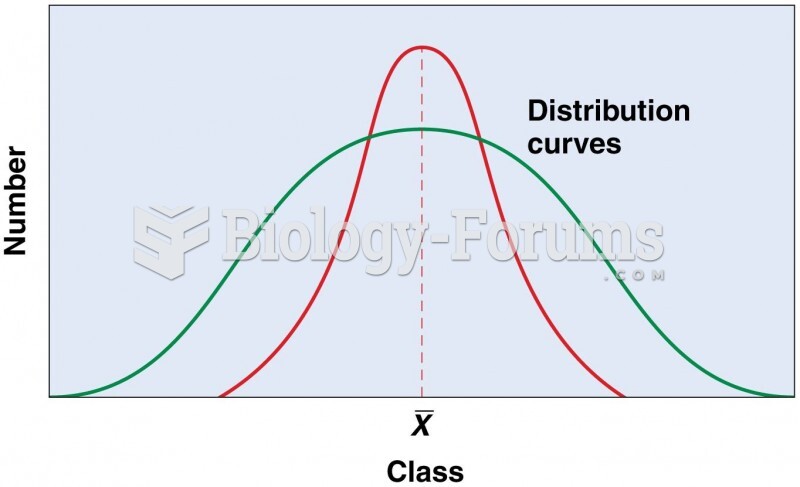
|
|
|
Medication errors are three times higher among children and infants than with adults.
It is important to read food labels and choose foods with low cholesterol and saturated trans fat. You should limit saturated fat to no higher than 6% of daily calories.
People with alcoholism are at a much greater risk of malnutrition than are other people and usually exhibit low levels of most vitamins (especially folic acid). This is because alcohol often takes the place of 50% of their daily intake of calories, with little nutritional value contained in it.
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.