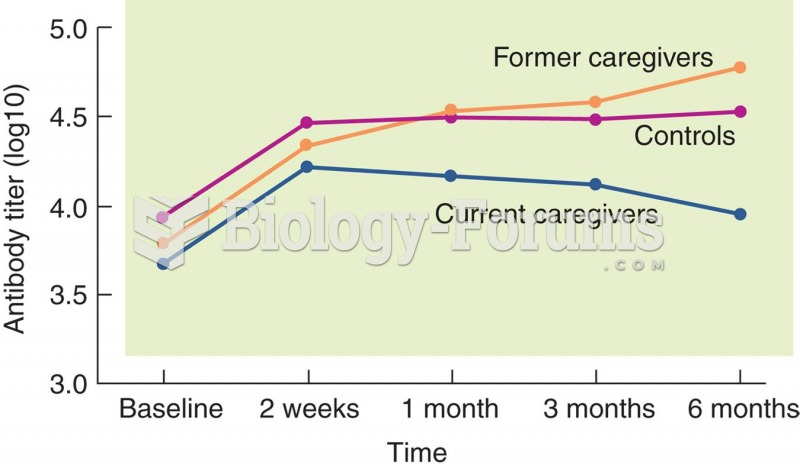
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Malaria mortality rates are falling. Increased malaria prevention and control measures have greatly improved these rates. Since 2000, malaria mortality rates have fallen globally by 60% among all age groups, and by 65% among children under age 5.
Did you know?
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
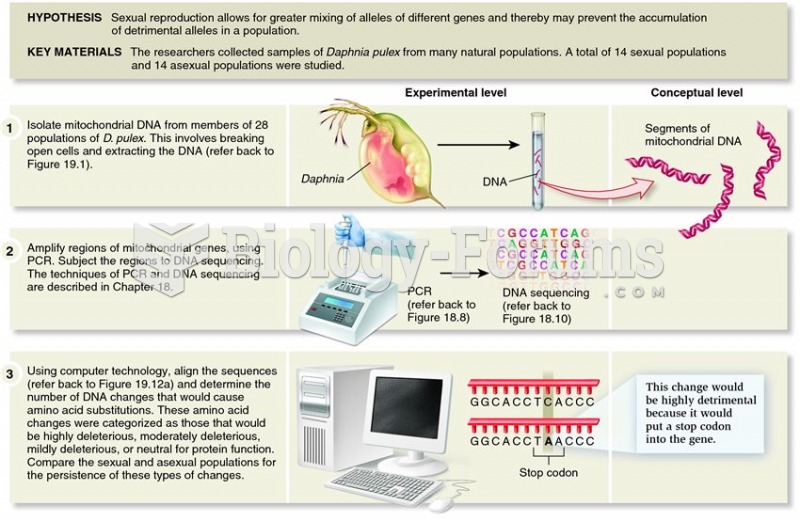 Paland and Lynch demonstrated the importance of sexual reproduction in reducing the frequency of mal
Paland and Lynch demonstrated the importance of sexual reproduction in reducing the frequency of mal
 Injecting an IV push (bolus) medication; C, another type of needleless syringe and needleless IV acc
Injecting an IV push (bolus) medication; C, another type of needleless syringe and needleless IV acc