|
|
|
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Although not all of the following muscle groups are commonly used, intramuscular injections may be given into the abdominals, biceps, calves, deltoids, gluteals, laterals, pectorals, quadriceps, trapezoids, and triceps.
Addicts to opiates often avoid treatment because they are afraid of withdrawal. Though unpleasant, with proper management, withdrawal is rarely fatal and passes relatively quickly.
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
 The 2012 U.S. presidential campaign rang with “get tough” cries to end Iran’s nuclear capacity and c
The 2012 U.S. presidential campaign rang with “get tough” cries to end Iran’s nuclear capacity and c
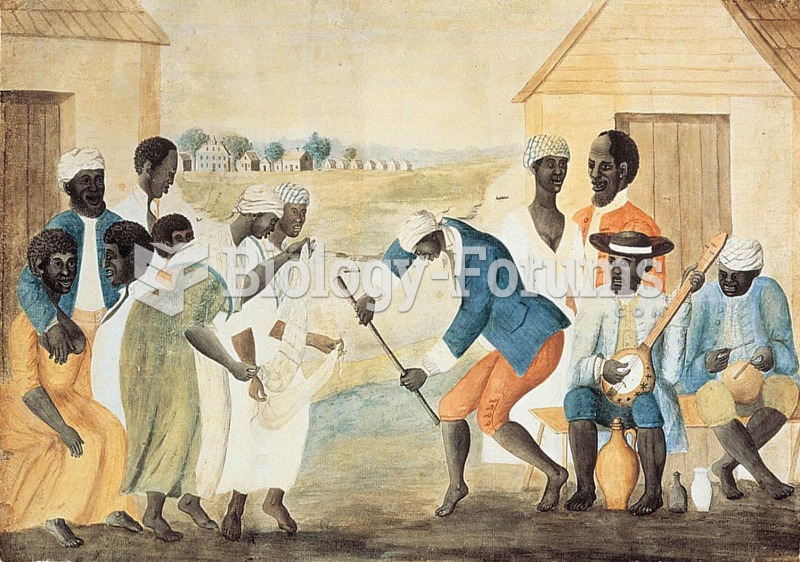 A depiction of slaves on a South Carolina plantation, around 1790. Likely of Yoruba descent, they pl
A depiction of slaves on a South Carolina plantation, around 1790. Likely of Yoruba descent, they pl