|
|
|
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
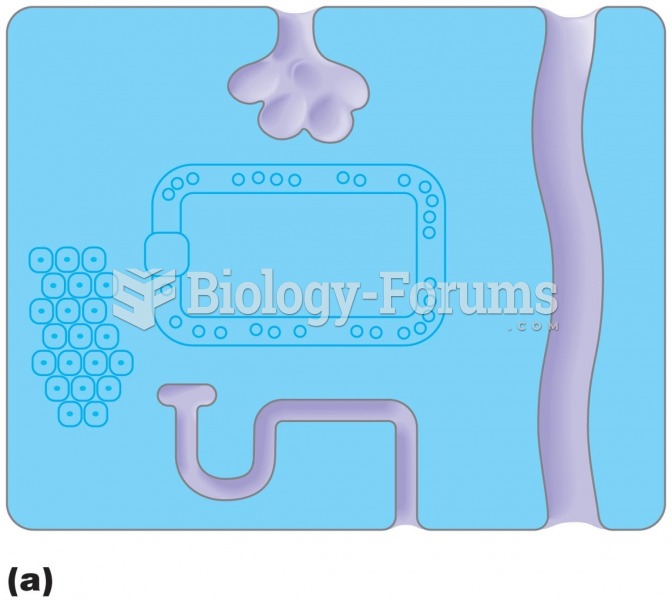
 The visual field as seen by a person with (a) glaucoma, (b) macular degeneration, and (c) cataracts.
The visual field as seen by a person with (a) glaucoma, (b) macular degeneration, and (c) cataracts.
 A typical eye wash station. A thorough flushing of the eyes with water is the first and often the ...
A typical eye wash station. A thorough flushing of the eyes with water is the first and often the ...