|
|
|
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
Although the Roman numeral for the number 4 has always been taught to have been "IV," according to historians, the ancient Romans probably used "IIII" most of the time. This is partially backed up by the fact that early grandfather clocks displayed IIII for the number 4 instead of IV. Early clockmakers apparently thought that the IIII balanced out the VIII (used for the number 8) on the clock face and that it just looked better.
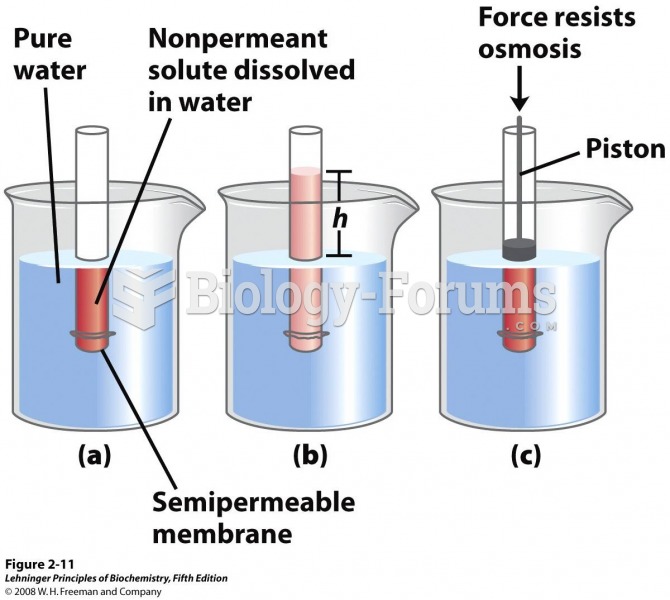
By definition, when a medication is administered intravenously, its bioavailability is 100%.
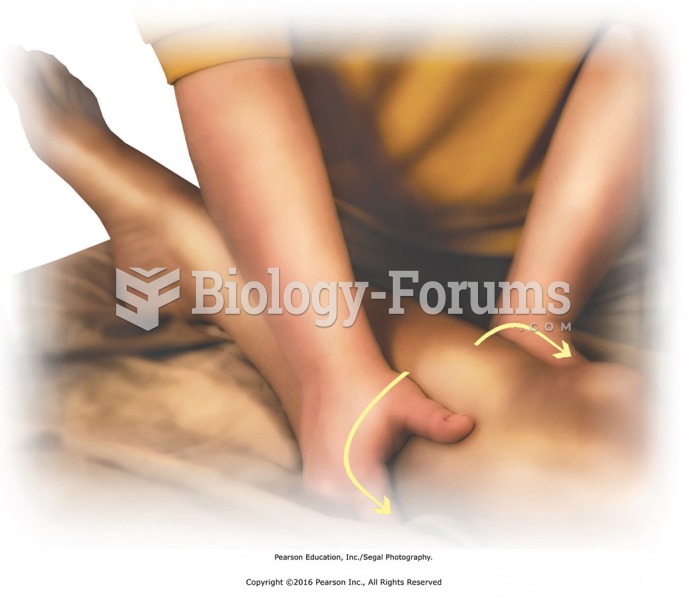
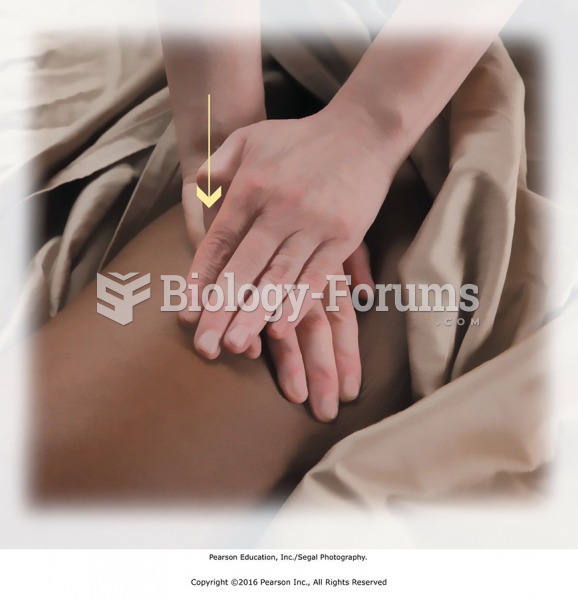 Compression using reinforced palm. The heel of the top hand is placed to avoid putting pressure on ...
Compression using reinforced palm. The heel of the top hand is placed to avoid putting pressure on ...
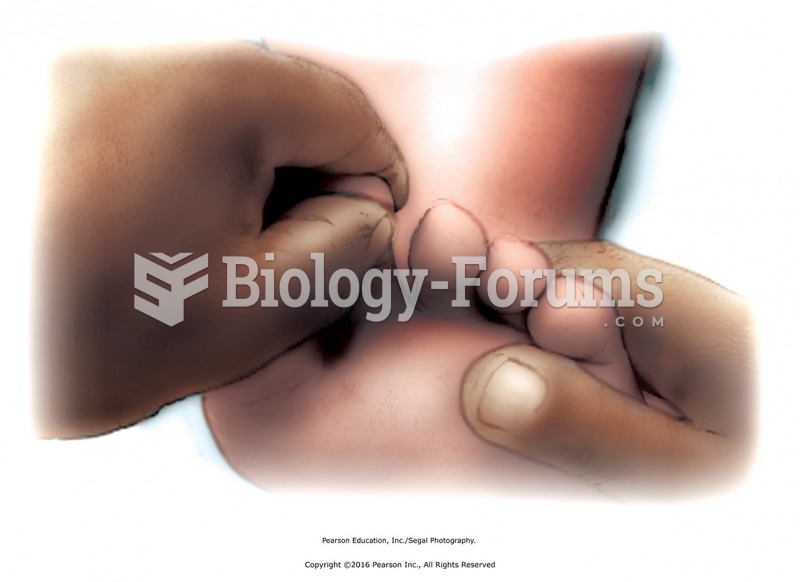
 Effleurage to transition to forearm-distal to proximal. Apply effleurage with moderate pressure to ...
Effleurage to transition to forearm-distal to proximal. Apply effleurage with moderate pressure to ...