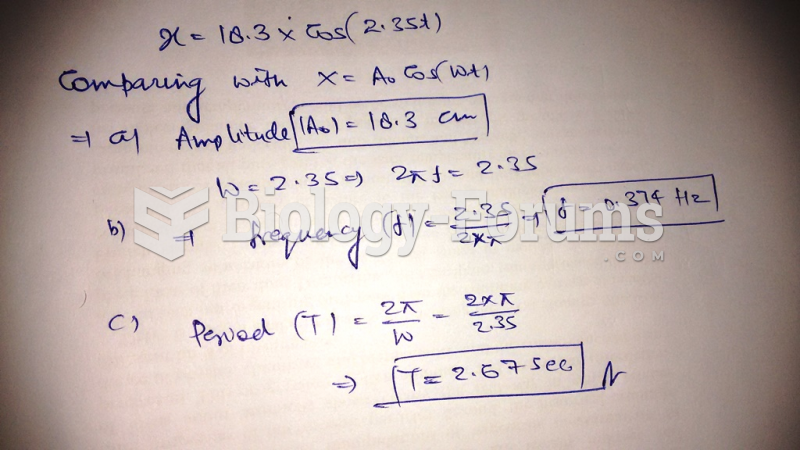
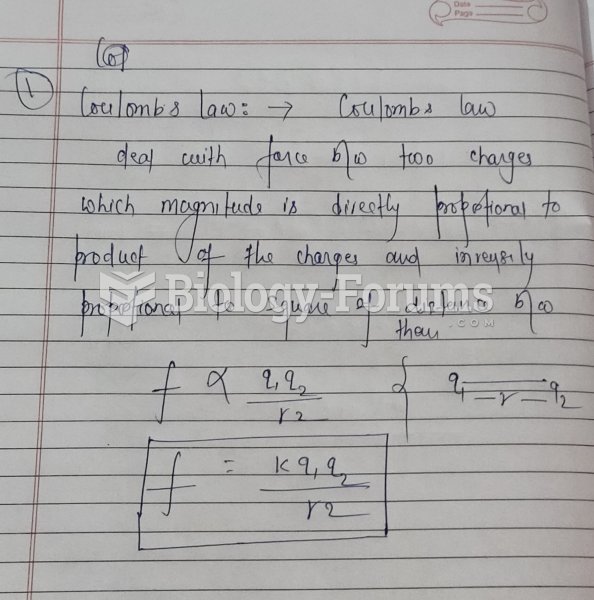
|
|
|
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.