Answer to Question 1
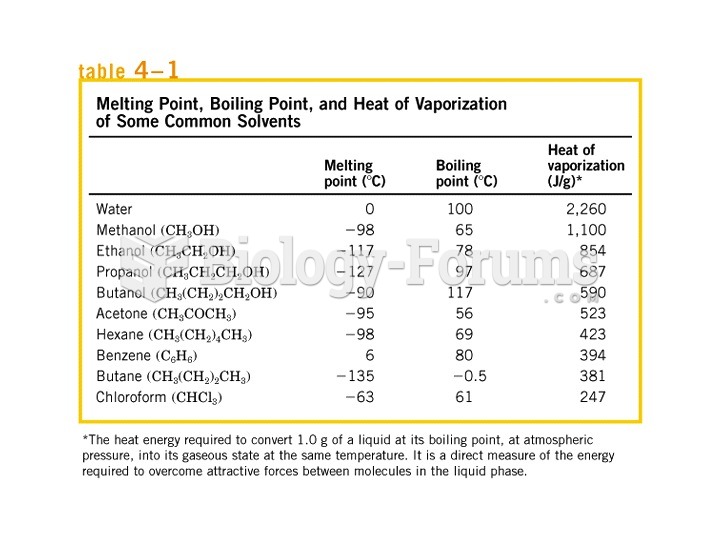
The flammability of PVC is largely dependant on the chlorine that is acting as a pendant group. As temperature raises these atoms begin to break off along with some of the carbon and hydrogen atoms. The difference is that the chlorine atoms act as an oxygen suppressant and deny the flame the oxygen it needs for flames to propagate. PE does not have these extinguishing atoms and will have a much greater ability to propagate the flames. The pendant group of chlorine will also increase both the modulus and strength of the PVC over the PE. This is largely due to the increased entanglement caused by the pendant group. The thermal
resistance of PVC will also increase with the chlorine group. The interlocking and entanglement between chains will help maintain the thermal stability. Because of the pendant chlorine the crystallinity will decrease and will increase the polymer's susceptibility to plasticizers. This particular pendant group is also a highly polar group. This will increase the polarity of the polymer as a whole. A highly polar polymer will always be more prone to adhesive bonding than a moderately inert polymer like polyethylene.
Answer to Question 2
Design, modeling, prototyping, material choice, processing selection, and production development should be some of the first engineering steps taken. The purpose of following a methodical stepwise plan is to eliminate errors and miscalculations that, while easily remedied in the design process, can be costly to fix once a part is in production.