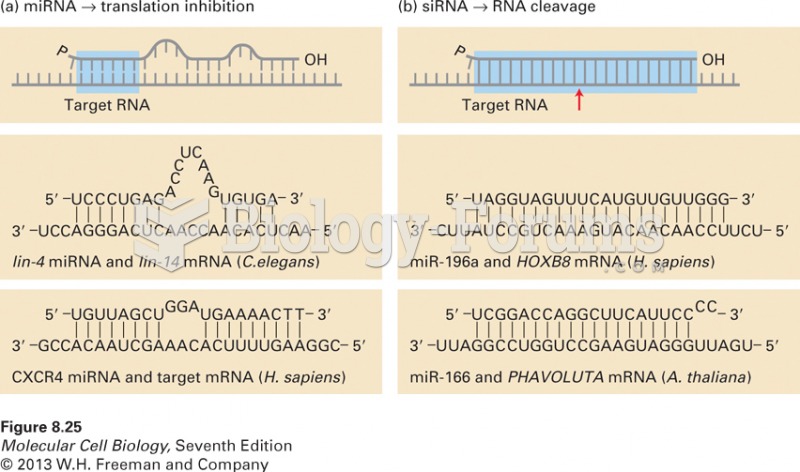
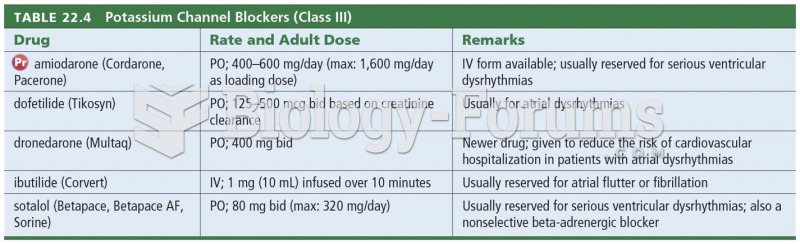
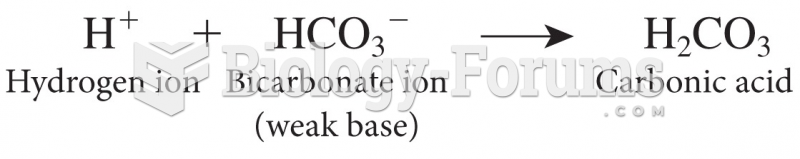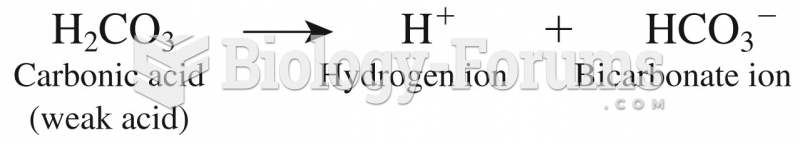When 1-bromobutane is reacted with the bulky base, potassium
tert-butoxide, in
tert-butyl alcohol, the major elimination product is:
A)
1-butene
B)
cis-2-butene
C)
trans-2-butene
D)
butyl
tert-butyl ether
E)
butyl alcohol
Question 2The major product of the following reaction is:


A)
I
B)
II
C)
III
D)
I and II
E)
there is no major product
Question 3The energy-reaction diagram below is for

A)
an S
N2 reaction only.
B)
an S
N1 reaction only.
C)
an E2 reaction only.
D)
an E1 reaction only.
E)
an S
N1 or E1 reaction.
F)
an S
N2 or E2 reaction
Question 4The structure below represents the transition state for the reaction of

A)
methanol with 2-bromopropene.
B)
methoxide with 2-bromopropane.
C)
sodium bromide with isopropyl methyl ether.
D)
methanol with 2-bromopropane.
E)
methoxide with 1-bromopropane.
Question 5
are most likely to react by
A)
a free radical chain mechanism.
B)
the S
N1 mechanism.
C)
the S
N2 mechanism.
D)
the E1 mechanism.
E)
the E2 mechanism.
Question 6CH
3CH
2C(CH
3)
2Br + (CH
3)
3CO
K
+ are most likely to react by
A)
a free radical chain mechanism.
B)
the S
N1 mechanism.
C)
the S
N2 mechanism.
D)
the E1 mechanism.
E)
the E2 mechanism.
Question 7The expected S
N2 reactivity order of the following is:



A)
2 > 1 > 3
B)
2 > 3 > 1
C)
1 > 2 > 3
D)
1 > 3 > 2
E)
3 > 1 > 2
Question 8The S
N1 mechanism for nucleophilic substitution reactions
A)
involves one step and occurs fastest with primary halides.
B)
involves one step and occurs fastest with tertiary halides.
C)
involves two steps and occurs fastest with tertiary halides.
D)
involves two steps and occurs fastest with primary halides.
E)
involves one step and occurs fastest with aromatic halides.