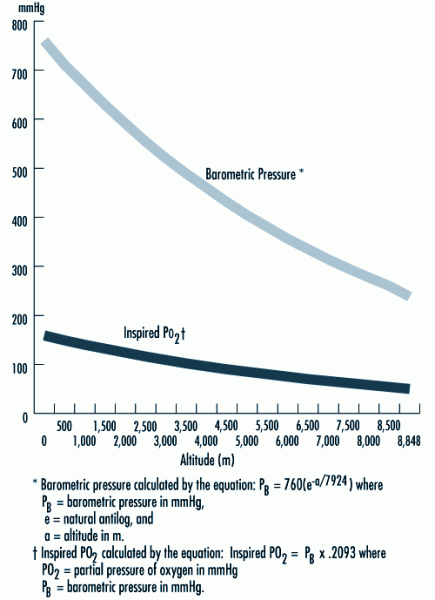
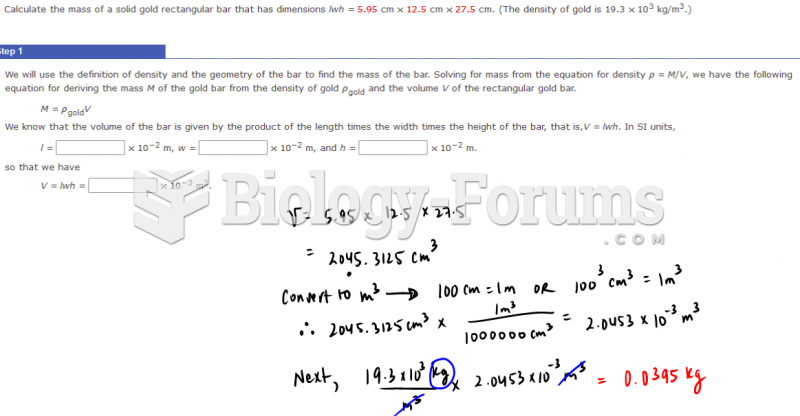
|
|
|
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
The senior population grows every year. Seniors older than 65 years of age now comprise more than 13% of the total population. However, women outlive men. In the 85-and-over age group, there are only 45 men to every 100 women.
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
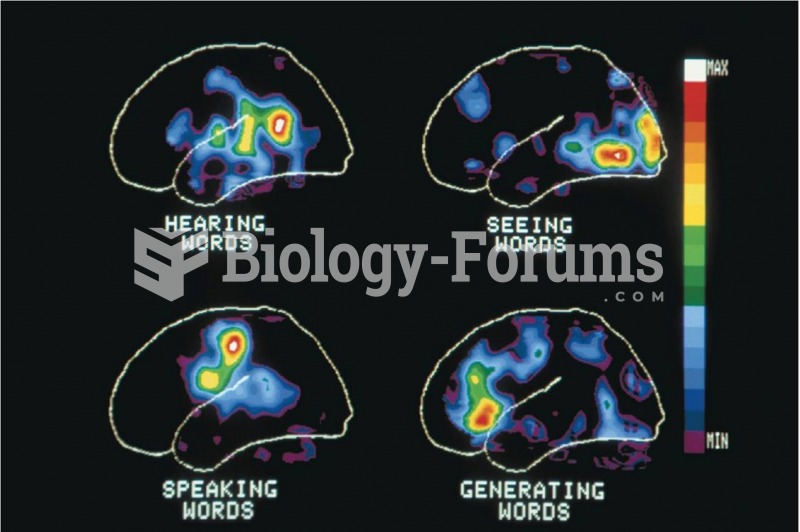 Is the self the same as the brain? Materialists contend that in the final analysis, mental states ...
Is the self the same as the brain? Materialists contend that in the final analysis, mental states ...
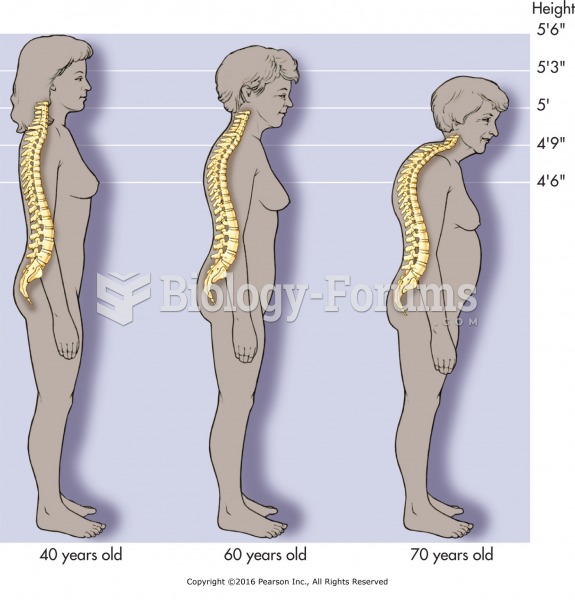 Spinal changes caused by osteoporosis. Use light pressure and gentle stretches only with frail or ...
Spinal changes caused by osteoporosis. Use light pressure and gentle stretches only with frail or ...
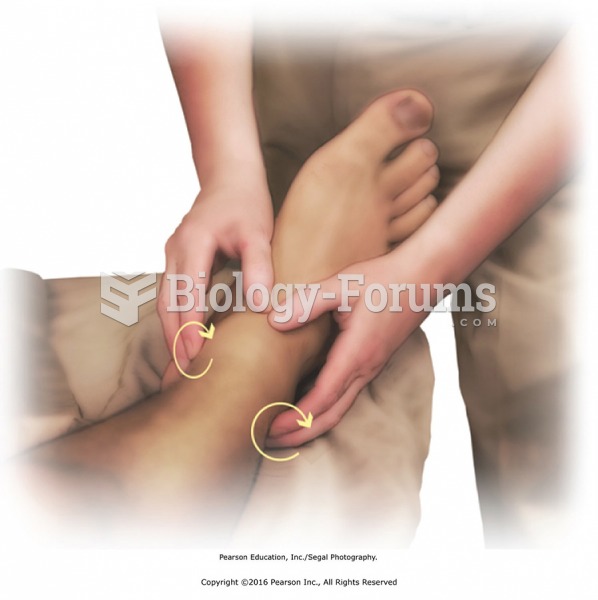 Circular friction around the ankle with the fingertips. Applying pressure with the fingertips, move ...
Circular friction around the ankle with the fingertips. Applying pressure with the fingertips, move ...