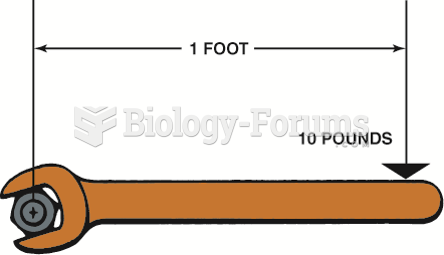
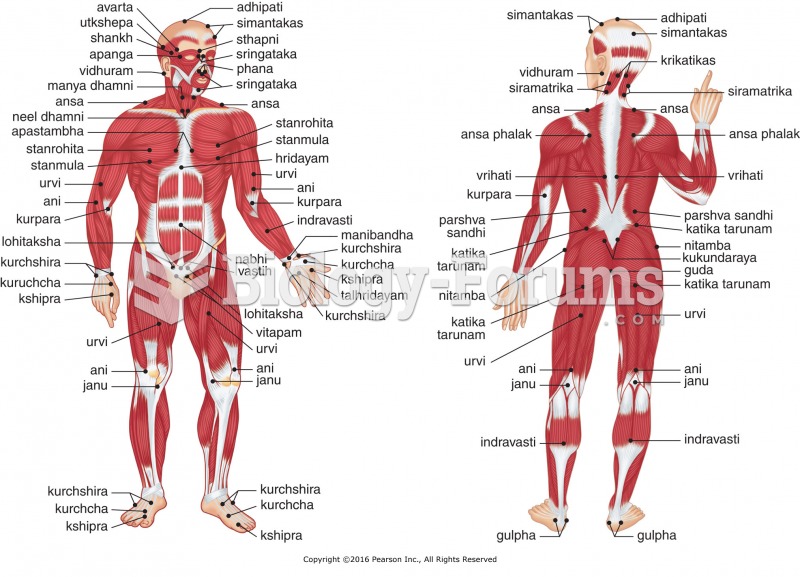
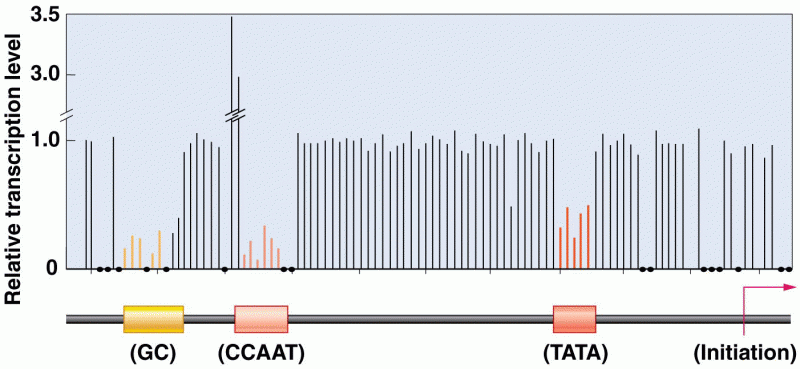
|
|
|
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.