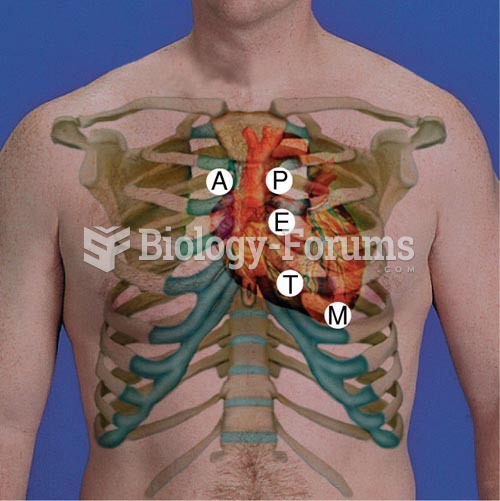
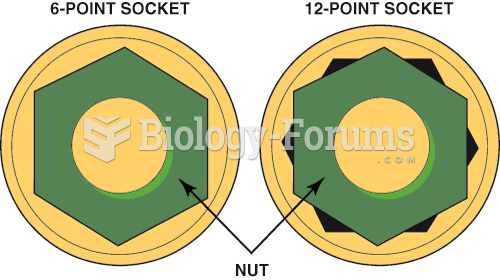
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
Did you know?
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.