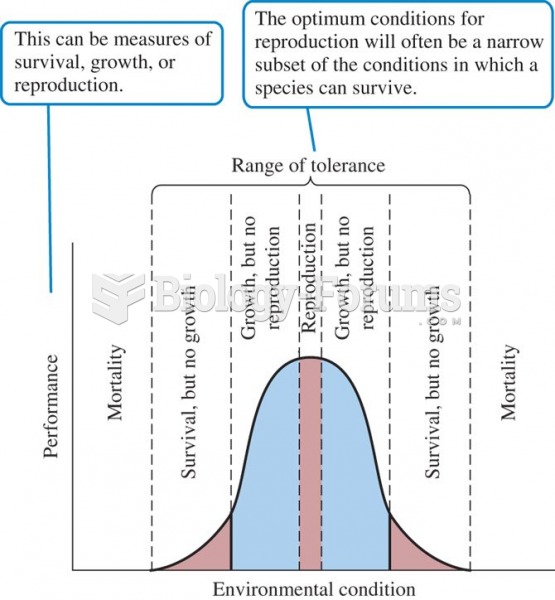
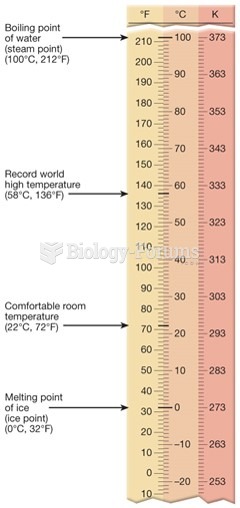
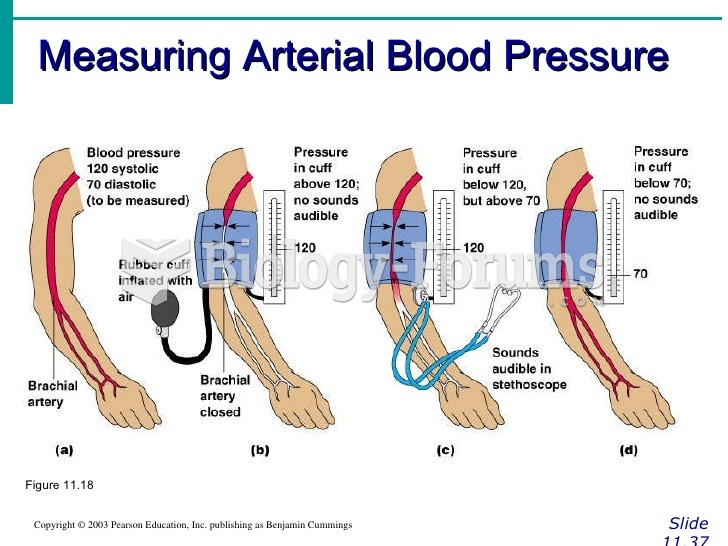
|
|
|
Did you know?
Children of people with alcoholism are more inclined to drink alcohol or use hard drugs. In fact, they are 400 times more likely to use hard drugs than those who do not have a family history of alcohol addiction.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.