|
|
|
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Asthma-like symptoms were first recorded about 3,500 years ago in Egypt. The first manuscript specifically written about asthma was in the year 1190, describing a condition characterized by sudden breathlessness. The treatments listed in this manuscript include chicken soup, herbs, and sexual abstinence.
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
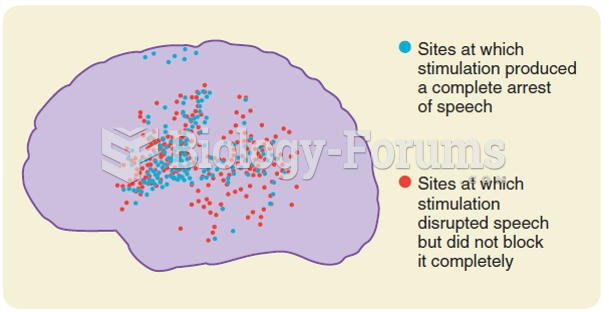 The wide distribution of left hemisphere sites where cortical stimulation either blocked speech or ...
The wide distribution of left hemisphere sites where cortical stimulation either blocked speech or ...
 Wide angle pose (upavistha konasana in hatha yoga), assisted by practitioner on the left during Thai ...
Wide angle pose (upavistha konasana in hatha yoga), assisted by practitioner on the left during Thai ...