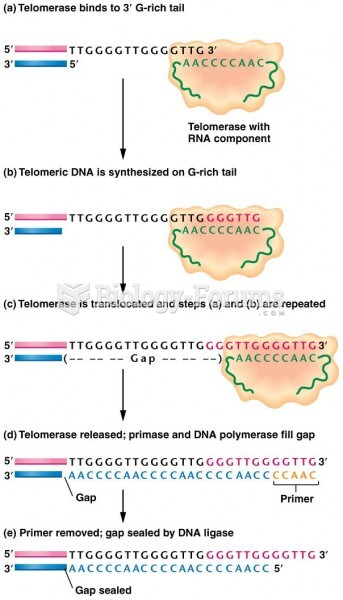
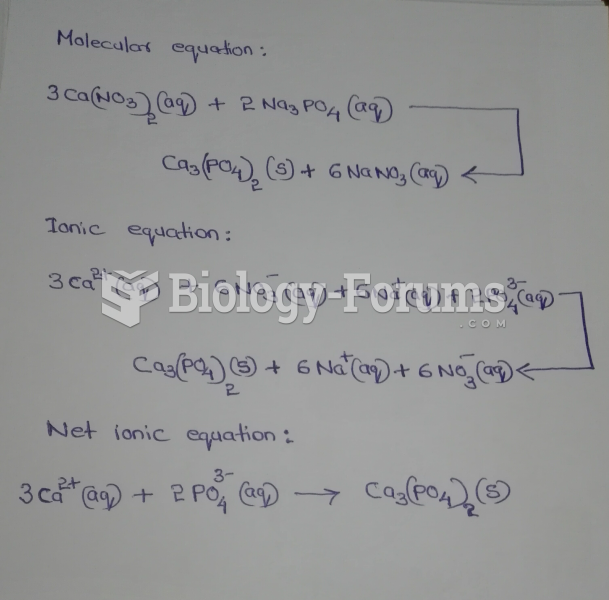
|
|
|
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
The first successful kidney transplant was performed in 1954 and occurred in Boston. A kidney from an identical twin was transplanted into his dying brother's body and was not rejected because it did not appear foreign to his body.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.