|
|
|
People who have myopia, or nearsightedness, are not able to see objects at a distance but only up close. It occurs when the cornea is either curved too steeply, the eye is too long, or both. This condition is progressive and worsens with time. More than 100 million people in the United States are nearsighted, but only 20% of those are born with the condition. Diet, eye exercise, drug therapy, and corrective lenses can all help manage nearsightedness.
Drug abusers experience the following scenario: The pleasure given by their drug (or drugs) of choice is so strong that it is difficult to eradicate even after years of staying away from the substances involved. Certain triggers may cause a drug abuser to relapse. Research shows that long-term drug abuse results in significant changes in brain function that persist long after an individual stops using drugs. It is most important to realize that the same is true of not just illegal substances but alcohol and tobacco as well.
Allergies play a major part in the health of children. The most prevalent childhood allergies are milk, egg, soy, wheat, peanuts, tree nuts, and seafood.
By definition, when a medication is administered intravenously, its bioavailability is 100%.
The training of an anesthesiologist typically requires four years of college, 4 years of medical school, 1 year of internship, and 3 years of residency.
 This bike actually flies—thanks to six horizontal propellers and a battery-powered motor. Changing ...
This bike actually flies—thanks to six horizontal propellers and a battery-powered motor. Changing ...
 Corrosion on a battery cable could be an indication that the battery is either being overcharged ...
Corrosion on a battery cable could be an indication that the battery is either being overcharged ...
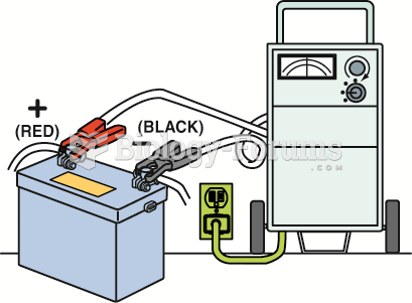 To use a battery charger, make sure the charger is connected to the battery before plugging in the ...
To use a battery charger, make sure the charger is connected to the battery before plugging in the ...




