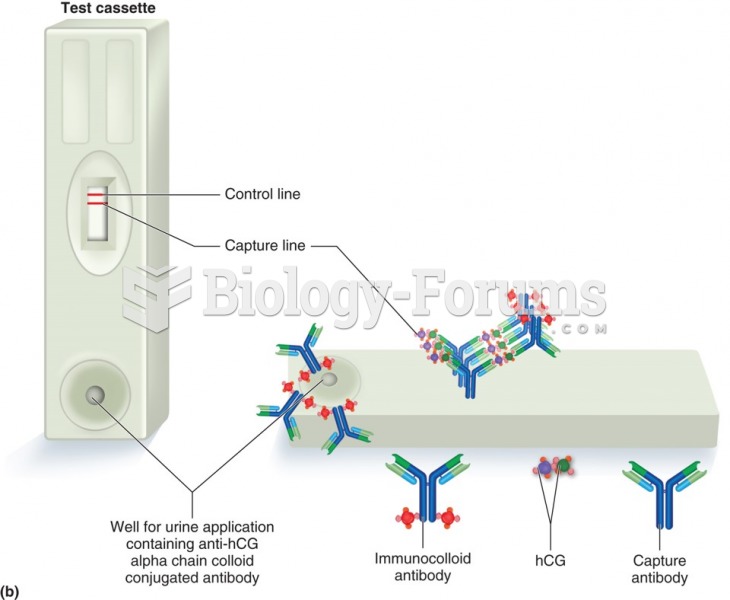
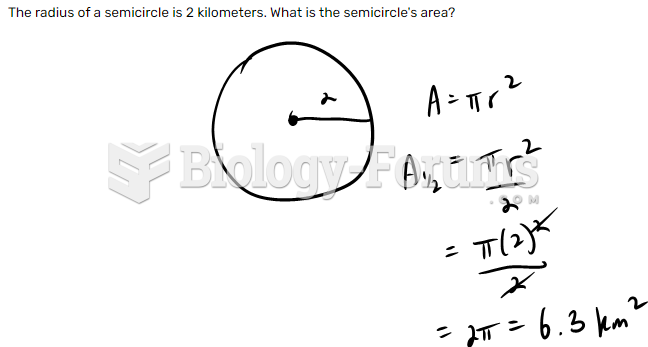
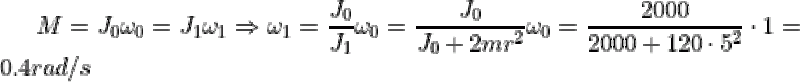|
|
|
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).
There are approximately 3 million unintended pregnancies in the United States each year.
Illness; diuretics; laxative abuse; hot weather; exercise; sweating; caffeine; alcoholic beverages; starvation diets; inadequate carbohydrate consumption; and diets high in protein, salt, or fiber can cause people to become dehydrated.
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
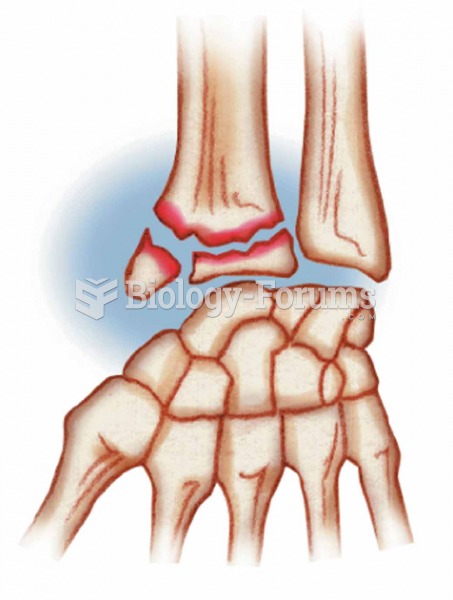 Colles’ A break in the distal portion of the radius; often the result of reaching out to cushion a f
Colles’ A break in the distal portion of the radius; often the result of reaching out to cushion a f
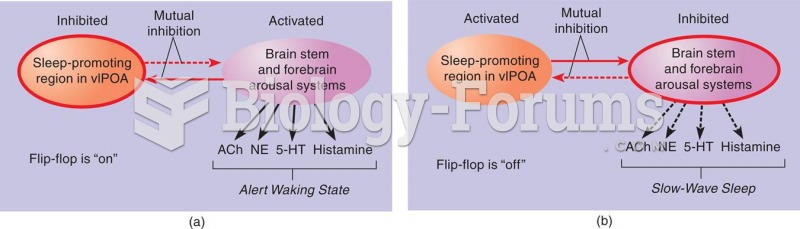 The Sleep/Waking Flip-Flop According to Saper et al. (2001), the major sleep-promoting region (the v
The Sleep/Waking Flip-Flop According to Saper et al. (2001), the major sleep-promoting region (the v