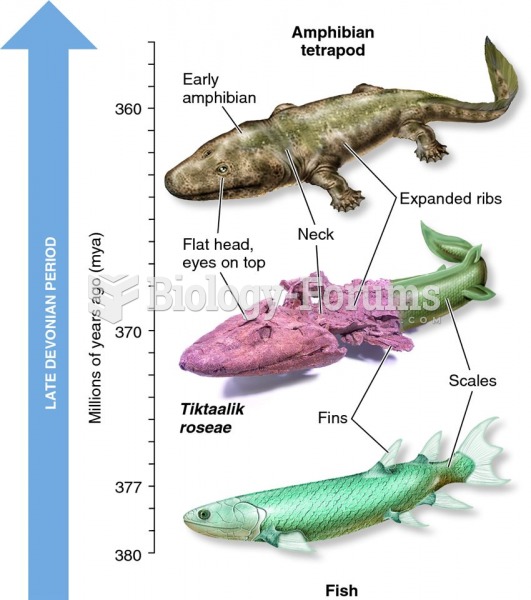
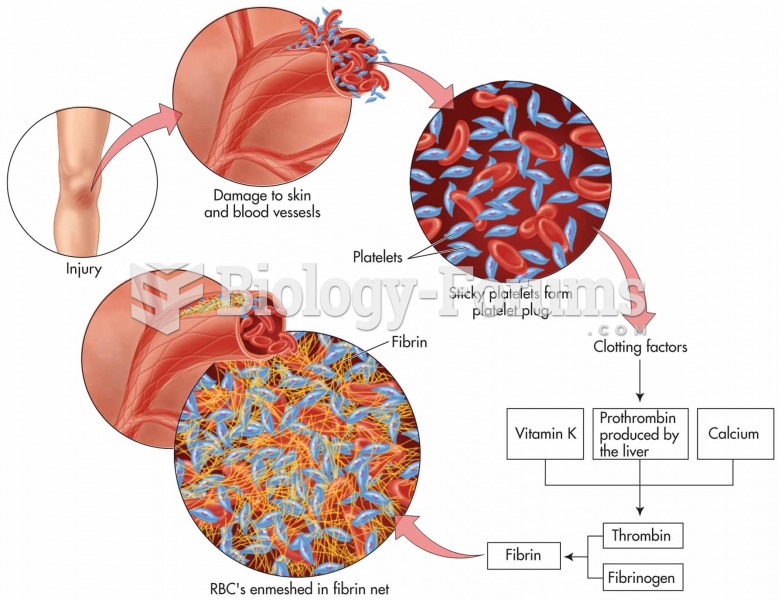
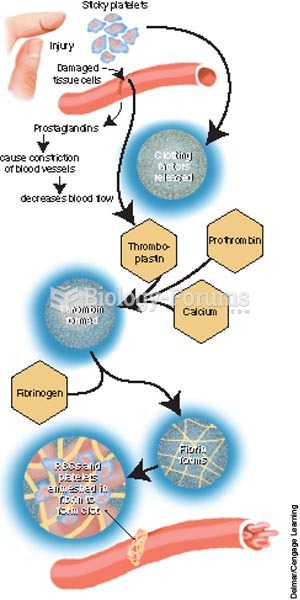
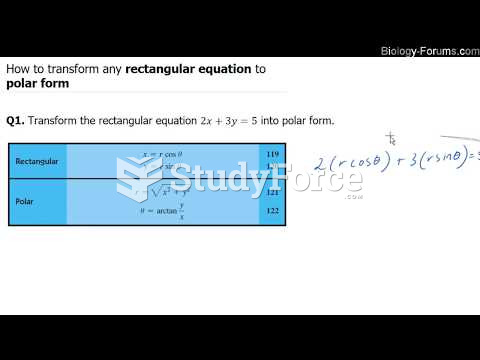
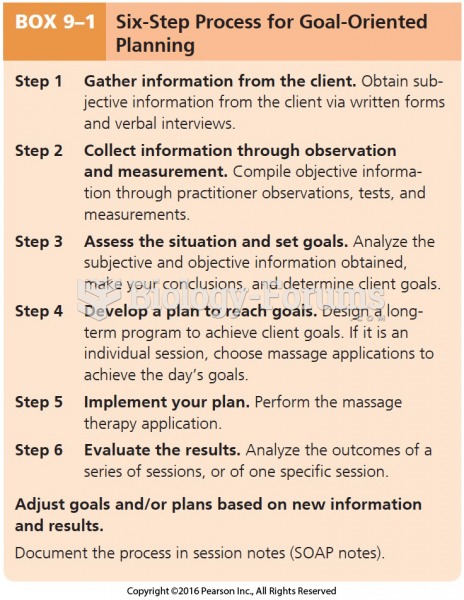
|
|
|
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
Alzheimer's disease affects only about 10% of people older than 65 years of age. Most forms of decreased mental function and dementia are caused by disuse (letting the mind get lazy).
Signs of depression include feeling sad most of the time for 2 weeks or longer; loss of interest in things normally enjoyed; lack of energy; sleep and appetite disturbances; weight changes; feelings of hopelessness, helplessness, or worthlessness; an inability to make decisions; and thoughts of death and suicide.
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.