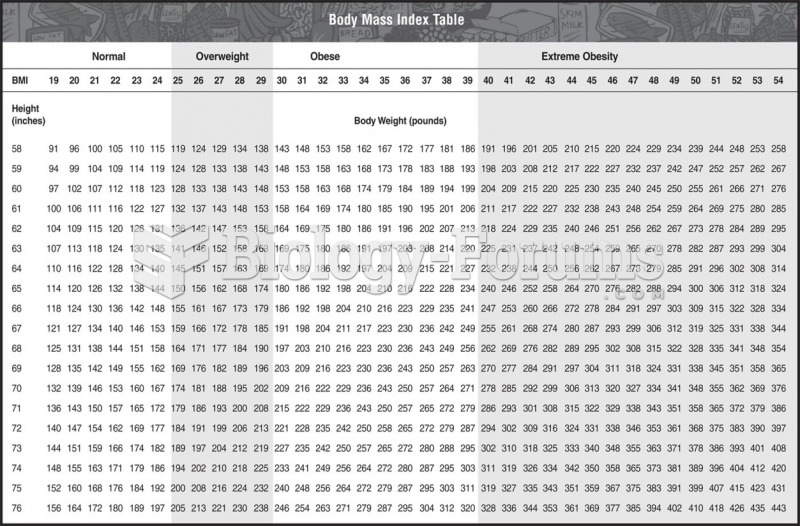
|
|
|
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.